
Distillation, or classical distillation, is the process of separating the components or substances from a liquid mixture by using selective boiling and condensation, usually inside an apparatus known as a still. Dry distillation is the heating of solid materials to produce gaseous products ; this may involve chemical changes such as destructive distillation or cracking. Distillation may result in essentially complete separation, or it may be a partial separation that increases the concentration of selected components; in either case, the process exploits differences in the relative volatility of the mixture's components. In industrial applications, distillation is a unit operation of practically universal importance, but is a physical separation process, not a chemical reaction. An installation used for distillation, especially of distilled beverages, is a distillery. Distillation includes the following applications:

Boiling is the rapid phase transition from liquid to gas or vapor; the reverse of boiling is condensation. Boiling occurs when a liquid is heated to its boiling point, so that the vapour pressure of the liquid is equal to the pressure exerted on the liquid by the surrounding atmosphere. Boiling and evaporation are the two main forms of liquid vapourization.
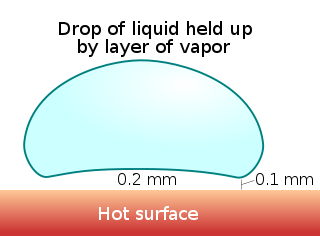
The Leidenfrost effect is a physical phenomenon in which a liquid, close to a surface that is significantly hotter than the liquid's boiling point, produces an insulating vapor layer that keeps the liquid from boiling rapidly. Because of this repulsive force, a droplet hovers over the surface, rather than making physical contact with it. The effect is named after the German doctor Johann Gottlob Leidenfrost, who described it in A Tract About Some Qualities of Common Water.

Heat transfer is a discipline of thermal engineering that concerns the generation, use, conversion, and exchange of thermal energy (heat) between physical systems. Heat transfer is classified into various mechanisms, such as thermal conduction, thermal convection, thermal radiation, and transfer of energy by phase changes. Engineers also consider the transfer of mass of differing chemical species, either cold or hot, to achieve heat transfer. While these mechanisms have distinct characteristics, they often occur simultaneously in the same system.
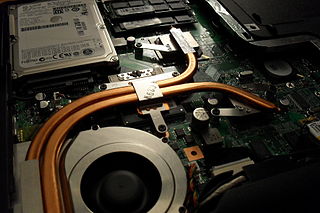
A heat pipe is a heat-transfer device that employs phase transition to transfer heat between two solid interfaces.

A chiller is a machine that removes heat from a liquid coolant via a vapor-compression, adsorption refrigeration, or absorption refrigeration cycles. This liquid can then be circulated through a heat exchanger to cool equipment, or another process stream. As a necessary by-product, refrigeration creates waste heat that must be exhausted to ambience, or for greater efficiency, recovered for heating purposes. Vapor compression chillers may use any of a number of different types of compressors. Most common today are the hermetic scroll, semi-hermetic screw, or centrifugal compressors. The condensing side of the chiller can be either air or water cooled. Even when liquid cooled, the chiller is often cooled by an induced or forced draft cooling tower. Absorption and adsorption chillers require a heat source to function.

Vacuum distillation or Distillation under reduced pressure is a type of distillation performed under reduced pressure, which allows the purification of compounds not readily distilled at ambient pressures or simply to save time or energy. This technique separates compounds based on differences in their boiling points. This technique is used when the boiling point of the desired compound is difficult to achieve or will cause the compound to decompose. Reduced pressures decrease the boiling point of compounds. The reduction in boiling point can be calculated using a temperature-pressure nomograph using the Clausius–Clapeyron relation.
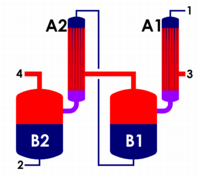
In chemical engineering, a multiple-effect evaporator is an apparatus for efficiently using the heat from steam to evaporate water. Water is boiled in a sequence of vessels, each held at a lower pressure than the last. Because the boiling temperature of water decreases as pressure decreases, the vapor boiled off in one vessel can be used to heat the next, and only the first vessel requires an external source of heat. The multiple-effect evaporator was invented by the American engineer Norbert Rillieux. Although he may have designed the apparatus during the 1820s and constructed a prototype in 1834, he did not build the first industrially practical evaporator until 1845. Originally designed for concentrating sugar in sugar cane juice, it has since become widely used in all industrial applications where large volumes of water must be evaporated, such as salt production and water desalination.

A falling film evaporator is an industrial device to concentrate solutions, especially with heat sensitive components. The evaporator is a special type of heat exchanger.
Multi-stage flash distillation (MSF) is a water desalination process that distills sea water by flashing a portion of the water into steam in multiple stages of what are essentially countercurrent heat exchangers. Current MSF facilities may have as many as 30 stages.
A shell-and-tube heat exchanger is a class of heat exchanger designs. It is the most common type of heat exchanger in oil refineries and other large chemical processes, and is suited for higher-pressure applications. As its name implies, this type of heat exchanger consists of a shell with a bundle of tubes inside it. One fluid runs through the tubes, and another fluid flows over the tubes to transfer heat between the two fluids. The set of tubes is called a tube bundle, and may be composed of several types of tubes: plain, longitudinally finned, etc.

Continuous distillation, a form of distillation, is an ongoing separation in which a mixture is continuously fed into the process and separated fractions are removed continuously as output streams. Distillation is the separation or partial separation of a liquid feed mixture into components or fractions by selective boiling and condensation. The process produces at least two output fractions. These fractions include at least one volatile distillate fraction, which has boiled and been separately captured as a vapor condensed to a liquid, and practically always a bottoms fraction, which is the least volatile residue that has not been separately captured as a condensed vapor.

Vacuum evaporation is the process of causing the pressure in a liquid-filled container to be reduced below the vapor pressure of the liquid, causing the liquid to evaporate at a lower temperature than normal. Although the process can be applied to any type of liquid at any vapor pressure, it is generally used to describe the boiling of water by lowering the container's internal pressure below standard atmospheric pressure and causing the water to boil at room temperature.
Economizers, or economisers (UK), are mechanical devices intended to reduce energy consumption, or to perform useful function such as preheating a fluid. The term economizer is used for other purposes as well. Boiler, power plant, heating, refrigeration, ventilating, and air conditioning (HVAC) uses are discussed in this article. In simple terms, an economizer is a heat exchanger.

Vapor-compression evaporation is the evaporation method by which a blower, compressor or jet ejector is used to compress, and thus, increase the pressure of the vapor produced. Since the pressure increase of the vapor also generates an increase in the condensation temperature, the same vapor can serve as the heating medium for its "mother" liquid or solution being concentrated, from which the vapor was generated to begin with. If no compression was provided, the vapor would be at the same temperature as the boiling liquid/solution, and no heat transfer could take place.

An evaporator is a type of heat exchanger device that facilitates evaporation by utilizing conductive and convective heat transfer to provide the necessary thermal energy for phase transition from liquid to vapor. Within evaporators, a circulating liquid is exposed to an atmospheric or reduced pressure environment, causing it to boil at a lower temperature compared to normal atmospheric boiling.
In fluid thermodynamics, nucleate boiling is a type of boiling that takes place when the surface temperature is hotter than the saturated fluid temperature by a certain amount but where the heat flux is below the critical heat flux. For water, as shown in the graph below, nucleate boiling occurs when the surface temperature is higher than the saturation temperature by between 10 and 30 °C. The critical heat flux is the peak on the curve between nucleate boiling and transition boiling. The heat transfer from surface to liquid is greater than that in film boiling.
Circulation evaporators are a type of evaporating unit designed to separate mixtures unable to be evaporated by a conventional evaporating unit. Circulation evaporation incorporates the use of both heat exchangers and flash separation units in conjunction with circulation of the solvent in order to remove liquid mixtures without conventional boiling. There are two types of Circulation Evaporation; Natural Circulation Evaporators and Forced Circulation Evaporators, both of which are still currently used in industry today, although forced Circulation systems, which have a circulation pump as opposed to natural systems with no driving force, have a much wider range of appropriate uses.
A rising film or vertical long tube evaporator is a type of evaporator that is essentially a vertical shell and tube heat exchanger. The liquid being evaporated is fed from the bottom into long tubes and heated with steam condensing on the outside of the tube from the shell side. This is to produce steam and vapour within the tube bringing the liquid inside to a boil. The vapour produced then presses the liquid against the walls of the tubes and causes the ascending force of this liquid. As more vapour is formed, the centre of the tube will have a higher velocity which forces the remaining liquid against the tube wall forming a thin film which moves upwards. This phenomenon of the rising film gives the evaporator its name.
Polymer devolatilization, also known as polymer degassing, is the process of removing low-molecular-weight components such as residual monomers, solvents, reaction by-products and water from polymers.













