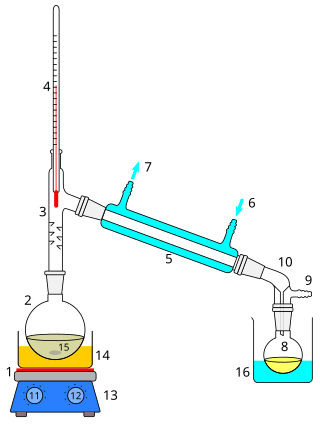
Distillation, also classical distillation, is the process of separating the component substances of a liquid mixture of two or more chemically discrete substances; the separation process is realized by way of the selective boiling of the mixture and the condensation of the vapors in a still.

Humidity is the concentration of water vapor present in the air. Water vapor, the gaseous state of water, is generally invisible to the human eye. Humidity indicates the likelihood for precipitation, dew, or fog to be present.
Water vapor, water vapour or aqueous vapor is the gaseous phase of water. It is one state of water within the hydrosphere. Water vapor can be produced from the evaporation or boiling of liquid water or from the sublimation of ice. Water vapor is transparent, like most constituents of the atmosphere. Under typical atmospheric conditions, water vapor is continuously generated by evaporation and removed by condensation. It is less dense than most of the other constituents of air and triggers convection currents that can lead to clouds and fog.

A boiler is a closed vessel in which fluid is heated. The fluid does not necessarily boil. The heated or vaporized fluid exits the boiler for use in various processes or heating applications, including water heating, central heating, boiler-based power generation, cooking, and sanitation.
Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. It uses distillation to fractionate. Generally the component parts have boiling points that differ by less than 25 °C (45 °F) from each other under a pressure of one atmosphere. If the difference in boiling points is greater than 25 °C, a simple distillation is typically used.

A dehumidifier is an air conditioning device which reduces and maintains the level of humidity in the air. This is done usually for health or thermal comfort reasons or to eliminate musty odor and to prevent the growth of mildew by extracting water from the air. It can be used for household, commercial, or industrial applications. Large dehumidifiers are used in commercial buildings such as indoor ice rinks and swimming pools, as well as manufacturing plants or storage warehouses. Typical air conditioning systems combine dehumidification with cooling, by operating cooling coils below the dewpoint and draining away the water that condenses.
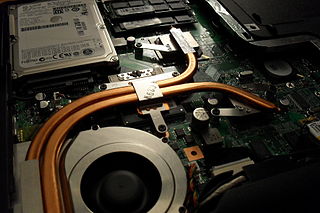
A heat pipe is a heat-transfer device that employs phase transition to transfer heat between two solid interfaces.

Superheated steam is steam at a temperature higher than its vaporization point at the absolute pressure where the temperature is measured.

A steam explosion is an explosion caused by violent boiling or flashing of water or ice into steam, occurring when water or ice is either superheated, rapidly heated by fine hot debris produced within it, or heated by the interaction of molten metals. Steam explosions are instances of explosive boiling. Pressure vessels, such as pressurized water (nuclear) reactors, that operate above atmospheric pressure can also provide the conditions for a steam explosion. The water changes from a solid or liquid to a gas with extreme speed, increasing dramatically in volume. A steam explosion sprays steam and boiling-hot water and the hot medium that heated it in all directions, creating a danger of scalding and burning.

Steam distillation is a separation process that consists of distilling water together with other volatile and non-volatile components. The steam from the boiling water carries the vapor of the volatiles to a condenser; both are cooled and return to the liquid or solid state, while the non-volatile residues remain behind in the boiling container.

In chemistry, volatility is a material quality which describes how readily a substance vaporizes. At a given temperature and pressure, a substance with high volatility is more likely to exist as a vapour, while a substance with low volatility is more likely to be a liquid or solid. Volatility can also describe the tendency of a vapor to condense into a liquid or solid; less volatile substances will more readily condense from a vapor than highly volatile ones. Differences in volatility can be observed by comparing how fast substances within a group evaporate when exposed to the atmosphere. A highly volatile substance such as rubbing alcohol will quickly evaporate, while a substance with low volatility such as vegetable oil will remain condensed. In general, solids are much less volatile than liquids, but there are some exceptions. Solids that sublimate such as dry ice or iodine can vaporize at a similar rate as some liquids under standard conditions.

Continuous distillation, a form of distillation, is an ongoing separation in which a mixture is continuously fed into the process and separated fractions are removed continuously as output streams. Distillation is the separation or partial separation of a liquid feed mixture into components or fractions by selective boiling and condensation. The process produces at least two output fractions. These fractions include at least one volatile distillate fraction, which has boiled and been separately captured as a vapor condensed to a liquid, and practically always a bottoms fraction, which is the least volatile residue that has not been separately captured as a condensed vapor.

A steam–electric power station is a power station in which the electric generator is steam-driven: water is heated, evaporates, and spins a steam turbine which drives an electric generator. After it passes through the turbine, the steam is condensed in a condenser. The greatest variation in the design of steam–electric power plants is due to the different fuel sources.
Economizers, or economisers (UK), are mechanical devices intended to reduce energy consumption, or to perform useful function such as preheating a fluid. The term economizer is used for other purposes as well. Boiler, power plant, heating, refrigeration, ventilating, and air conditioning (HVAC) may all use economizers. In simple terms, an economizer is a heat exchanger.

Vapor-compression evaporation is the evaporation method by which a blower, compressor or jet ejector is used to compress, and thus, increase the pressure of the vapor produced. Since the pressure increase of the vapor also generates an increase in the condensation temperature, the same vapor can serve as the heating medium for its "mother" liquid or solution being concentrated, from which the vapor was generated to begin with. If no compression was provided, the vapor would be at the same temperature as the boiling liquid/solution, and no heat transfer could take place.

An evaporator is a type of heat exchanger device that facilitates evaporation by utilizing conductive and convective heat transfer, which provides the necessary thermal energy for phase transition from liquid to vapour. Within evaporators, a circulating liquid is exposed to an atmospheric or reduced pressure environment causing it to boil at a lower temperature compared to normal atmospheric boiling.

In chemistry, a condenser is laboratory apparatus used to condense vapors – that is, turn them into liquids – by cooling them down.

In systems involving heat transfer, a condenser is a heat exchanger used to condense a gaseous substance into a liquid state through cooling. In doing so, the latent heat is released by the substance and transferred to the surrounding environment. Condensers are used for efficient heat rejection in many industrial systems. Condensers can be made according to numerous designs and come in many sizes ranging from rather small (hand-held) to very large. For example, a refrigerator uses a condenser to get rid of heat extracted from the interior of the unit to the outside air.
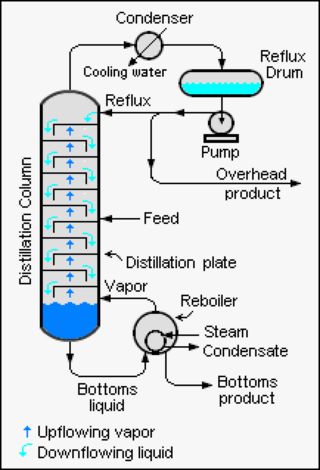
Reflux is a technique involving the condensation of vapors and the return of this condensate to the system from which it originated. It is used in industrial and laboratory distillations. It is also used in chemistry to supply energy to reactions over a long period of time.

The Hygroscopic cycle is a thermodynamic cycle converting thermal energy into mechanical power by the means of a steam turbine. It is similar to the Rankine cycle using water as the motive fluid but with the novelty of introducing salts and their hygroscopic properties for the condensation. The salts are desorbed in the boiler or steam generator, where clean steam is released and superheated in order to be expanded and generate power through the steam turbine. Boiler blowdown with the concentrated hygroscopic compounds is used thermally to pre-heat the steam turbine condensate, and as reflux in the steam-absorber.

















