Related Research Articles

The European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of pharmaceutical products. Prior to 2004, it was known as the European Agency for the Evaluation of Medicinal Products or European Medicines Evaluation Agency (EMEA).

Ichthammol or ammonium bituminosulfonate, also known as black ointment, is a medication derived from sulfur-rich oil shale. It is used as a treatment for different skin diseases, including eczema and psoriasis. It is applied on the skin as an ointments, most commonly containing 10% or 20% ichthammol.
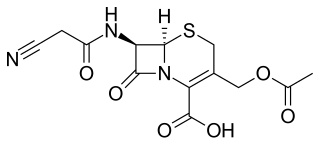
Cefacetrile is a broad-spectrum first generation cephalosporin antibiotic effective in gram-positive and gram-negative bacterial infections. It is a bacteriostatic antibiotic. Cefacetrile is marketed under the trade names Celospor, Celtol, and Cristacef, and as Vetimast for the treatment of mammary infections in lactating cows.

Trilostane, sold under the brand name Vetoryl among others, is a medication which has been used in the treatment of Cushing's syndrome, Conn's syndrome, and postmenopausal breast cancer in humans. It was withdrawn for use in humans in the United States in the 1990s but was subsequently approved for use in veterinary medicine in the 2000s to treat Cushing's syndrome in dogs. It is taken by mouth.
Ustekinumab, sold under the brand name Stelara among others, is a monoclonal antibody medication developed by Janssen Pharmaceuticals, for the treatment of Crohn's disease, ulcerative colitis, plaque psoriasis and psoriatic arthritis, targeting both IL-12 and IL-23.

Tepoxalin, sold under the brand name Zubrin among others, is a non-steroidal anti-flammatory drug (NSAIDs) generally used in veterinary medicine to reduce swelling in animals with osteoarthritis. In rare circumstances, tepoxalin can also be used in human pharmacology to relieve pain caused by musculoskeletal conditions such as arthritis and hip dysplasia.

Narcobarbital (Pronarcon) is a barbiturate derivative developed in 1932 by Carl Heinrich Friedrich Boedecker and Heinrich Gruber Schoneberg, assignors to the firm J. D. Riedel-E. de Haën AG, Berlin, Germany. Later, in 1937, may, was patented in United States. It is an N-methylated derivative of propallylonal and has similar sedative effects. It is still used in veterinary medicine for inducing surgical anaesthesia.
The Committee for Medicinal Products for Human Use (CHMP), formerly known as the Committee for Proprietary Medicinal Products (CPMP), is the European Medicines Agency's committee responsible for elaborating the agency's opinions on all issues regarding medicinal products for human use.
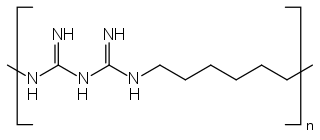
Polyhexanide is a polymer used as a disinfectant and antiseptic. In dermatological use, it is spelled polihexanide (INN) and sold under various brand names. PHMB has been shown to be effective against Pseudomonas aeruginosa, Staphylococcus aureus, Escherichia coli, Candida albicans, Aspergillus brasiliensis, enterococci, and Klebsiella pneumoniae. Polihexanide, sold under the brand name Akantior is a medication used for the treatment of Acanthamoeba keratitis.
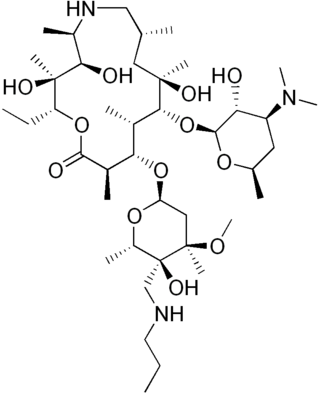
Tulathromycin, sold under the brand name Draxxin among others, is a macrolide antibiotic used to treat bovine respiratory disease in cattle and swine respiratory disease in pigs.

Pradofloxacin, sold under the brand name Veraflox among others, is a third-generation enhanced spectrum veterinary antibiotic of the fluoroquinolone class. It was developed by Elanco Animal Health GmbH and received approval from the European Commission in April 2011, for prescription-only use in veterinary medicine for the treatment of bacterial infections in dogs and cats.
ATCvet code QIImmunologicals is a section of the Anatomical Therapeutic Chemical Classification System for veterinary medicinal products, a system of alphanumeric codes developed by the World Health Organization (WHO) for the classification of drugs and other medical products for veterinary use.
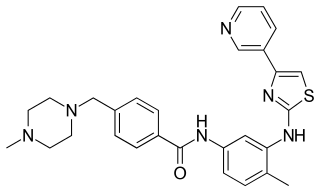
Masitinib is a tyrosine-kinase inhibitor used in the treatment of mast cell tumours in animals, specifically dogs. Since its introduction in November 2008 it has been distributed under the commercial name Masivet. It has been available in Europe since the second part of 2009. Masitinib has been studied for several human conditions including melanoma, multiple myeloma, gastrointestinal cancer, pancreatic cancer, Alzheimer disease, multiple sclerosis, rheumatoid arthritis, mastocytosis, amyotrophic lateral sclerosis and COVID-19.
Obiltoxaximab, sold under the brand name Anthim among others, is a monoclonal antibody medication designed for the treatment of exposure to Bacillus anthracis spores.
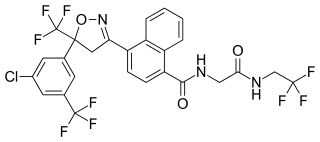
Afoxolaner (INN) is an insecticide and acaricide that belongs to the isoxazoline chemical compound group.
Tagraxofusp, sold under the brand name Elzonris, is an anti-cancer medication for the treatment of blastic plasmacytoid dendritic cell neoplasm (BPDCN).
The Spanish Agency of Medicines and Medical Devices is a regulatory and autonomous agency of the Government of Spain that acts as the highest sanitary authority in the country in terms of medical safety on medicines, health products, cosmetics and personal care products.

Lotilaner, sold under the brand name Xdemvy, is an ectoparasiticide (anti-parasitic) medication used for the treatment of blepharitis caused by infestation by Demodex. It is used as an eye drop.
Fenofibrate/pravastatin, sold under the brand name Pravafenix, is a combination medication used for the treatment of hypercholesterolemia in adults whose low-density lipoprotein (LDL) cholesterol is already being controlled with pravastatin alone but who still need to improve their cholesterol levels and to reduce their levels of triglycerides. It contains fenofibrate and pravastatin. It is taken by mouth.
Terbinafine/betamethasone acetate, sold under the brand name Duotic is a veterinary medication used for the treatment of otitis externa in dogs. It is a fixed dose combination of terbinafine, an antifungal; and betamethasone acetate, a glucocorticosteroid anti-inflammatory.