
Distillation, or classical distillation, is the process of separating the components or substances from a liquid mixture by using selective boiling and condensation. Dry distillation is the heating of solid materials to produce gaseous products. Dry distillation may involve chemical changes such as destructive distillation or cracking and is not discussed under this article. Distillation may result in essentially complete separation, or it may be a partial separation that increases the concentration of selected components in the mixture. In either case, the process exploits differences in the relative volatility of the mixture's components. In industrial applications, distillation is a unit operation of practically universal importance, but it is a physical separation process, not a chemical reaction.

Desalination is a process that takes away mineral components from saline water. More generally, desalination refers to the removal of salts and minerals from a target substance, as in soil desalination, which is an issue for agriculture. Saltwater is desalinated to produce water suitable for human consumption or irrigation. The by-product of the desalination process is brine. Desalination is used on many seagoing ships and submarines. Most of the modern interest in desalination is focused on cost-effective provision of fresh water for human use. Along with recycled wastewater, it is one of the few rainfall-independent water resources.
Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. It uses distillation to fractionate. Generally the component parts have boiling points that differ by less than 25 °C (45 °F) from each other under a pressure of one atmosphere. If the difference in boiling points is greater than 25 °C, a simple distillation is typically used. It is used to refine crude oil.

A chiller is a machine that removes heat from a liquid coolant via a vapor-compression, adsorption refrigeration, or absorption refrigeration cycles. This liquid can then be circulated through a heat exchanger to cool equipment, or another process stream. As a necessary by-product, refrigeration creates waste heat that must be exhausted to ambience, or for greater efficiency, recovered for heating purposes. Vapor compression chillers may use any of a number of different types of compressors. Most common today are the hermetic scroll, semi-hermetic screw, or centrifugal compressors. The condensing side of the chiller can be either air or water cooled. Even when liquid cooled, the chiller is often cooled by an induced or forced draft cooling tower. Absorption and adsorption chillers require a heat source to function.

Vacuum distillation is distillation performed under reduced pressure, which allows the purification of compounds not readily distilled at ambient pressures or simply to save time or energy. This technique separates compounds based on differences in their boiling points. This technique is used when the boiling point of the desired compound is difficult to achieve or will cause the compound to decompose. Reduced pressures decrease the boiling point of compounds. The reduction in boiling point can be calculated using a temperature-pressure nomograph using the Clausius–Clapeyron relation.
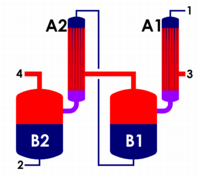
A multiple-effect evaporator, as defined in chemical engineering, is an apparatus for efficiently using the heat from steam to evaporate water. In a multiple-effect evaporator, water is boiled in a sequence of vessels, each held at a lower pressure than the last. Because the boiling temperature of water decreases as pressure decreases, the vapor boiled off in one vessel can be used to heat the next, and only the first vessel requires an external source of heat. While in theory, evaporators may be built with an arbitrarily large number of stages, evaporators with more than four stages are rarely practical except in systems where the liquor is the desired product such as in chemical recovery systems where up to seven effects are used.
Multi-stage flash distillation (MSF) is a water desalination process that distills sea water by flashing a portion of the water into steam in multiple stages of what are essentially countercurrent heat exchangers. Current MSF facilities may have as many as 30 stages.
Solar desalination is a desalination technique powered by solar energy. The two common methods are direct (thermal) and indirect (photovoltaic).

An absorption refrigerator is a refrigerator that uses a heat source to provide the energy needed to drive the cooling process. The system uses two coolants, the first of which performs evaporative cooling and is then absorbed into the second coolant; heat is needed to reset the two coolants to their initial states. The principle can also be used to air-condition buildings using the waste heat from a gas turbine or water heater. Using waste heat from a gas turbine makes the turbine very efficient because it first produces electricity, then hot water, and finally, air-conditioning—trigeneration. Absorption refrigerators are commonly used in recreational vehicles (RVs), campers, and caravans because the heat required to power them can be provided by a propane fuel burner, by a low-voltage DC electric heater or by a mains-powered electric heater. Unlike more common vapor-compression refrigeration systems, an absorption refrigerator can be produced with no moving parts other than the coolants.
An atmospheric water generator (AWG), also known as a hydro panel or hydropanel, is a device that extracts water from humid ambient air, producing potable water. Water vapor in the air can be extracted by multiple techniques, including condensation - cooling the air below its dew point, exposing the air to desiccants, or pressurizing the air. AWGs are useful where potable water is difficult to obtain, because water is always present in ambient air.

Vapor-compression evaporation is the evaporation method by which a blower, compressor or jet ejector is used to compress, and thus, increase the pressure of the vapor produced. Since the pressure increase of the vapor also generates an increase in the condensation temperature, the same vapor can serve as the heating medium for its "mother" liquid or solution being concentrated, from which the vapor was generated to begin with. If no compression was provided, the vapor would be at the same temperature as the boiling liquid/solution, and no heat transfer could take place.
An evaporator is a device used to turn the liquid form of a chemical substance, such as water, into its gaseous form - vapor, therefore changing the substance's state of matter. In this process, the liquid is evaporated, or vaporized.
A solar-powered desalination unit produces potable water from saline water through direct or indirect methods of desalination powered by sunlight. Solar energy is the most promising renewable energy source due to its ability to drive the more popular thermal desalination systems directly through solar collectors and to drive physical and chemical desalination systems indirectly through photovoltaic cells.
A seawater greenhouse is a greenhouse structure that enables the growth of crops and the production of fresh water in arid regions which constitute about one third of the earth's land area. This in response to the global water scarcity and peak water and the salt-infecting soil. The system uses seawater and solar energy. It uses a similar structure to the pad-and-fan greenhouse, but with additional evaporators and condensers. The seawater is pumped into the greenhouse to create a cool and humid environment, the optimal conditions for the cultivation of temperate crops. The freshwater is produced in a condensed state created by the solar desalination principle, which removes salt and impurities. Finally, the remaining humidified air is expelled from the greenhouse and used to improve growing conditions for outdoor plants.
Multiple-effect distillation or multi-effect distillation (MED) is a distillation process often used for sea water desalination. It consists of multiple stages or "effects". In each stage the feed water is heated by steam in tubes, usually by spraying saline water onto them. Some of the water evaporates, and this steam flows into the tubes of the next stage (effect), heating and evaporating more water. Each stage essentially reuses the energy from the previous stage, with successively lower temperatures and pressures after each one. There are different configurations, such as forward-feed, backward-feed, etc. Additionally, between stages this steam uses some heat to preheat incoming saline water.
Vapor-compression desalination (VC) refers to a distillation process where the evaporation of sea or saline water is obtained by the application of heat delivered by compressed vapor.

An evaporator, distiller or distilling apparatus is a piece of ship's equipment used to produce fresh drinking water from sea water by distillation. As fresh water is bulky, may spoil in storage, and is an essential supply for any long voyage, the ability to produce more fresh water in mid-ocean is important for any ship.
Membrane distillation (MD) is a thermally driven separation process in which separation is driven by phase change. A hydrophobic membrane presents a barrier for the liquid phase, allowing the vapour phase to pass through the membrane's pores. The driving force of the process is a partial vapour pressure difference commonly triggered by a temperature difference.

Zero Liquid Discharge(ZLD) is a treatment process designed to remove all the liquid waste from a system. The focus of ZLD is to reduce wastewater economically and produce clean water that is suitable for reuse, thereby saving money and being beneficial to the environment. ZLD systems employ advanced wastewater/desalination treatment technologies to purify and recycle virtually all of the wastewater produced.
The low-temperature distillation (LTD) technology is the first implementation of the direct spray distillation (DSD) process. The first large-scale units are now in operation for desalination. The process was first developed by scientists at the University of Applied Sciences in Switzerland, focusing on low-temperature distillation in vacuum conditions, from 2000 to 2005.








