Conductor or conduction may refer to:
Conductor or conduction may refer to:
A semiconductor is a material that has an electrical conductivity value falling between that of a conductor, such as copper, and an insulator, such as glass. Its resistivity generally falls as its temperature rises; metals behave in the opposite way. In many cases their conducting properties may be altered in useful ways by introducing impurities ("doping") into the crystal structure. When two differently doped regions exist in the same crystal, a semiconductor junction is created. The behavior of charge carriers, which include electrons, ions, and electron holes, at these junctions is the basis of diodes, transistors, and most modern electronics. Some examples of semiconductors are silicon, germanium, gallium arsenide, and elements near the so-called "metalloid staircase" on the periodic table. After silicon, gallium arsenide is the second-most common semiconductor and is used in laser diodes, solar cells, microwave-frequency integrated circuits, and others. Silicon is a critical element for fabricating most electronic circuits.

Ohm's law states that the electric current through a conductor between two points is directly proportional to the voltage across the two points. Introducing the constant of proportionality, the resistance, one arrives at the three mathematical equations used to describe this relationship:
The thermal conductivity of a material is a measure of its ability to conduct heat. It is commonly denoted by , , or and is measured in W·m−1·K−1.
Electrical resistivity is a fundamental specific property of a material that measures its electrical resistance or how strongly it resists electric current. A low resistivity indicates a material that readily allows electric current. Resistivity is commonly represented by the Greek letter ρ (rho). The SI unit of electrical resistivity is the ohm-metre (Ω⋅m). For example, if a 1 m3 solid cube of material has sheet contacts on two opposite faces, and the resistance between these contacts is 1 Ω, then the resistivity of the material is 1 Ω⋅m.
Thermal conduction is the diffusion of thermal energy (heat) within one material or between materials in contact. The higher temperature object has molecules with more kinetic energy; collisions between molecules distributions this kinetic energy until an object has the same thermal energy throughout. Thermal conductivity, frequently represented by k, is a property that relates the rate of heat loss per unit area of a material to its rate of change of temperature. Essentially, it is a value that accounts for any property of the material that could change the way it conducts heat. Heat spontaneously flows along a temperature gradient. For example, heat is conducted from the hotplate of an electric stove to the bottom of a saucepan in contact with it. In the absence of an opposing external driving energy source, within a body or between bodies, temperature differences decay over time, and thermal equilibrium is approached, temperature becoming more uniform.
Solid-state physics is the study of rigid matter, or solids, through methods such as solid-state chemistry, quantum mechanics, crystallography, electromagnetism, and metallurgy. It is the largest branch of condensed matter physics. Solid-state physics studies how the large-scale properties of solid materials result from their atomic-scale properties. Thus, solid-state physics forms a theoretical basis of materials science. Along with solid-state chemistry, it also has direct applications in the technology of transistors and semiconductors.
Resistance may refer to:

Heat transfer is a discipline of thermal engineering that concerns the generation, use, conversion, and exchange of thermal energy (heat) between physical systems. Heat transfer is classified into various mechanisms, such as thermal conduction, thermal convection, thermal radiation, and transfer of energy by phase changes. Engineers also consider the transfer of mass of differing chemical species, either cold or hot, to achieve heat transfer. While these mechanisms have distinct characteristics, they often occur simultaneously in the same system.

In physics and electrical engineering, a conductor is an object or type of material that allows the flow of charge in one or more directions. Materials made of metal are common electrical conductors. The flow of negatively charged electrons generates electric current, positively charged holes, and positive or negative ions in some cases.
In solid state physics, a charge carrier is a particle or quasiparticle that is free to move, carrying an electric charge, especially the particles that carry electric charges in electrical conductors. Examples are electrons, ions and holes. In a conducting medium, an electric field can exert force on these free particles, causing a net motion of the particles through the medium; this is what constitutes an electric current. The electron and the proton are the elementary charge carriers, each carrying one elementary charge (e), of the same magnitude and opposite sign.

A semimetal is a material with a small energy overlap between the bottom of the conduction band and the top of the valence band, but they do not overlap in momentum space. According to electronic band theory, solids can be classified as insulators, semiconductors, semimetals, or metals. In insulators and semiconductors the filled valence band is separated from an empty conduction band by a band gap. For insulators, the magnitude of the band gap is larger than that of a semiconductor. Because of the slight overlap between the conduction and valence bands, semimetals have no band gap and a small density of states at the Fermi level. A metal, by contrast, has an appreciable density of states at the Fermi level because the conduction band is partially filled.
In solid-state physics, the free electron model is a quantum mechanical model for the behaviour of charge carriers in a metallic solid. It was developed in 1927, principally by Arnold Sommerfeld, who combined the classical Drude model with quantum mechanical Fermi–Dirac statistics and hence it is also known as the Drude–Sommerfeld model.
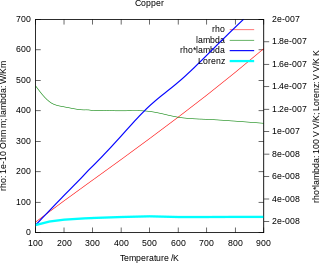
In physics, the Wiedemann–Franz law states that the ratio of the electronic contribution of the thermal conductivity (κ) to the electrical conductivity (σ) of a metal is proportional to the temperature (T).
An intrinsic semiconductor, also called a pure semiconductor, undoped semiconductor or i-type semiconductor, is a semiconductor without any significant dopant species present. The number of charge carriers is therefore determined by the properties of the material itself instead of the amount of impurities. In intrinsic semiconductors the number of excited electrons and the number of holes are equal: n = p. This may be the case even after doping the semiconductor, though only if it is doped with both donors and acceptors equally. In this case, n = p still holds, and the semiconductor remains intrinsic, though doped. This means that some conductors are both intrinsic as well as extrinsic but only if n is equal to p.
In physics, thermal contact conductance is the study of heat conduction between solid or liquid bodies in thermal contact. The thermal contact conductance coefficient, , is a property indicating the thermal conductivity, or ability to conduct heat, between two bodies in contact. The inverse of this property is termed thermal contact resistance.
In solid-state physics, the Poole–Frenkel effect is a model describing the mechanism of trap-assisted electron transport in an electrical insulator. It is named after Yakov Frenkel, who published on it in 1938, extending the theory previously developed by H. H. Poole.

Solid-state ionics is the study of ionic-electronic mixed conductor and fully ionic conductors and their uses. Some materials that fall into this category include inorganic crystalline and polycrystalline solids, ceramics, glasses, polymers, and composites. Solid-state ionic devices, such as solid oxide fuel cells, can be much more reliable and long-lasting, especially under harsh conditions, than comparable devices with fluid electrolytes.
In heat transfer, thermal engineering, and thermodynamics, thermal conductance and thermal resistance are fundamental concepts that describe the ability of materials or systems to conduct heat and the opposition they offer to the heat current. The ability to manipulate these properties allows engineers to control temperature gradient, prevent thermal shock, and maximize the efficiency of thermal systems. Furthermore, these principles find applications in a multitude of fields, including materials science, mechanical engineering, electronics, and energy management. Knowledge of these principles is crucial in various scientific, engineering, and everyday applications, from designing efficient temperature control, thermal insulation, and thermal management in industrial processes to optimizing the performance of electronic devices.

Copper has been used in electrical wiring since the invention of the electromagnet and the telegraph in the 1820s. The invention of the telephone in 1876 created further demand for copper wire as an electrical conductor.

In solid-state physics, the valence band and conduction band are the bands closest to the Fermi level, and thus determine the electrical conductivity of the solid. In nonmetals, the valence band is the highest range of electron energies in which electrons are normally present at absolute zero temperature, while the conduction band is the lowest range of vacant electronic states. On a graph of the electronic band structure of a semiconducting material, the valence band is located below the Fermi level, while the conduction band is located above it.