
Evaporation is a type of vaporization that occurs on the surface of a liquid as it changes into the gas phase. High concentration of the evaporating substance in the surrounding gas significantly slows down evaporation, such as when humidity affects rate of evaporation of water. When the molecules of the liquid collide, they transfer energy to each other based on how they collide. When a molecule near the surface absorbs enough energy to overcome the vapor pressure, it will escape and enter the surrounding air as a gas. When evaporation occurs, the energy removed from the vaporized liquid will reduce the temperature of the liquid, resulting in evaporative cooling.

Humidity is the concentration of water vapor present in the air. Water vapor, the gaseous state of water, is generally invisible to the human eye. Humidity indicates the likelihood for precipitation, dew, or fog to be present.

William Hyde Wollaston was an English chemist and physicist who is famous for discovering the chemical elements palladium and rhodium. He also developed a way to process platinum ore into malleable ingots.

A hygrometer is an instrument used to measure the amount of water vapor in air, in soil, or in confined spaces. Humidity measurement instruments usually rely on measurements of some other quantities such as temperature, pressure, mass, a mechanical or electrical change in a substance as moisture is absorbed. By calibration and calculation, these measured quantities can lead to a measurement of humidity. Modern electronic devices use temperature of condensation, or changes in electrical capacitance or resistance to measure humidity differences. A crude hygrometer was invented by Leonardo da Vinci in 1480. Major leaps came forward during the 1600s; Francesco Folli invented a more practical version of the device, while Robert Hooke improved a number of meteorological devices including the hygrometer. A more modern version was created by Swiss polymath Johann Heinrich Lambert in 1755. Later, in the year 1783, Swiss physicist and Geologist Horace Bénédict de Saussure invented the first hygrometer using human hair to measure humidity.

Hydraulic shock is a pressure surge or wave caused when a fluid in motion, usually a liquid but sometimes also a gas is forced to stop or change direction suddenly; a momentum change. This phenomenon commonly occurs when a valve closes suddenly at an end of a pipeline system, and a pressure wave propagates in the pipe.
The Mpemba effect is the name given to the observation that a liquid which is initially hot can freeze faster than the same liquid which begins cold, under otherwise similar conditions. There is disagreement about its theoretical basis and the parameters required to produce the effect.

A cloud chamber, also known as a Wilson cloud chamber, is a particle detector used for visualizing the passage of ionizing radiation.
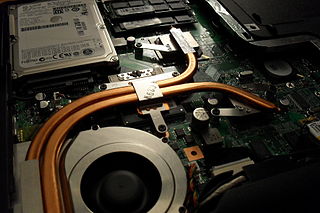
A heat pipe is a heat-transfer device that employs phase transition to transfer heat between two solid interfaces.

An evaporative cooler is a device that cools air through the evaporation of water. Evaporative cooling differs from other air conditioning systems, which use vapor-compression or absorption refrigeration cycles. Evaporative cooling exploits the fact that water will absorb a relatively large amount of heat in order to evaporate. The temperature of dry air can be dropped significantly through the phase transition of liquid water to water vapor (evaporation). This can cool air using much less energy than refrigeration. In extremely dry climates, evaporative cooling of air has the added benefit of conditioning the air with more moisture for the comfort of building occupants.

Cloud physics is the study of the physical processes that lead to the formation, growth and precipitation of atmospheric clouds. These aerosols are found in the troposphere, stratosphere, and mesosphere, which collectively make up the greatest part of the homosphere. Clouds consist of microscopic droplets of liquid water, tiny crystals of ice, or both. Cloud droplets initially form by the condensation of water vapor onto condensation nuclei when the supersaturation of air exceeds a critical value according to Köhler theory. Cloud condensation nuclei are necessary for cloud droplets formation because of the Kelvin effect, which describes the change in saturation vapor pressure due to a curved surface. At small radii, the amount of supersaturation needed for condensation to occur is so large, that it does not happen naturally. Raoult's law describes how the vapor pressure is dependent on the amount of solute in a solution. At high concentrations, when the cloud droplets are small, the supersaturation required is smaller than without the presence of a nucleus.

A cooling tower is a device that rejects waste heat to the atmosphere through the cooling of a coolant stream, usually a water stream to a lower temperature. Cooling towers may either use the evaporation of water to remove process heat and cool the working fluid to near the wet-bulb air temperature or, in the case of dry cooling towers, rely solely on air to cool the working fluid to near the dry-bulb air temperature using radiators.

Psychrometrics is the field of engineering concerned with the physical and thermodynamic properties of gas-vapor mixtures.

Drinking birds, also known as insatiable birdies, dunking birds, drinky birds, water birds, dipping birds, and “SippyChickens” are toy heat engines that mimic the motions of a bird drinking from a water source. They are sometimes incorrectly considered examples of a perpetual motion device.

Thermosiphon is a method of passive heat exchange, based on natural convection, which circulates a fluid without the necessity of a mechanical pump. Thermosiphoning is used for circulation of liquids and volatile gases in heating and cooling applications such as heat pumps, water heaters, boilers and furnaces. Thermosiphoning also occurs across air temperature gradients such as those utilized in a wood fire chimney or solar chimney.

An absorption refrigerator is a refrigerator that uses a heat source to provide the energy needed to drive the cooling process. The system uses two coolants, the first of which performs evaporative cooling and is then absorbed into the second coolant; heat is needed to reset the two coolants to their initial states. The principle can also be used to air-condition buildings using the waste heat from a gas turbine or water heater. Using waste heat from a gas turbine makes the turbine very efficient because it first produces electricity, then hot water, and finally, air-conditioning—trigeneration. Absorption refrigerators are commonly used in recreational vehicles (RVs), campers, and caravans because the heat required to power them can be provided by a propane fuel burner, by a low-voltage DC electric heater or by a mains-powered electric heater. Unlike more common vapor-compression refrigeration systems, an absorption refrigerator has no moving parts.

Deposition is the phase transition in which gas transforms into solid without passing through the liquid phase. Deposition is a thermodynamic process. The reverse of deposition is sublimation and hence sometimes deposition is called desublimation.

A thermal expansion valve or thermostatic expansion valve is a component in vapor-compression refrigeration and air conditioning systems that controls the amount of refrigerant released into the evaporator and is intended to regulate the superheat of the refrigerant that flows out of the evaporator to a steady value. Although often described as a "thermostatic" valve, an expansion valve is not able to regulate the evaporator's temperature to a precise value. The evaporator's temperature will vary only with the evaporating pressure, which will have to be regulated through other means.

A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a (nearly) constant volume independent of pressure. As such, it is one of the four fundamental states of matter, and is the only state with a definite volume but no fixed shape. A liquid is made up of tiny vibrating particles of matter, such as atoms, held together by intermolecular bonds. Like a gas, a liquid is able to flow and take the shape of a container. Most liquids resist compression, although others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly constant density. A distinctive property of the liquid state is surface tension, leading to wetting phenomena. Water is by far the most common liquid on Earth.

A hand boiler or love meter is a glass sculpture used as an experimental tool to demonstrate vapour-liquid equilibrium, or as a collector's item to whimsically "measure love." It consists of a lower bulb containing a volatile liquid and a mixture of gases that is connected usually by a twisting glass tube that connects to an upper or "receiving" glass bulb.

Ground freezing is a construction technique used in circumstances where soil needs to be stabilized so it will not collapse next to excavations, or to prevent contaminants spilled into soil from being leached away. Ground freezing has been used for at least one hundred years.



















