Related Research Articles
Sodium dodecyl sulfate (SDS) or sodium lauryl sulfate (SLS), sometimes written sodium laurilsulfate, is an organic compound with the formula CH3(CH2)11OSO3Na and structure H3C−(CH2)11−O−S(=O)2−O−Na+. It is an anionic surfactant used in many cleaning and hygiene products. This compound is the sodium salt of the 12-carbon organosulfate. Its hydrocarbon tail combined with a polar "headgroup" give the compound amphiphilic properties that make it useful as a detergent. SDS is also component of mixtures produced from inexpensive coconut and palm oils. SDS is a common component of many domestic cleaning, personal hygiene and cosmetic, pharmaceutical, and food products, as well as of industrial and commercial cleaning and product formulations.

Citric acid is an organic compound with the chemical formula HOC(CO2H)(CH2CO2H)2. It is a colorless weak organic acid. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the metabolism of all aerobic organisms.

A detergent is a surfactant or a mixture of surfactants with cleansing properties when in dilute solutions. There are a large variety of detergents, a common family being the alkylbenzene sulfonates, which are soap-like compounds that are more soluble in hard water, because the polar sulfonate is less likely than the polar carboxylate to bind to calcium and other ions found in hard water.

Surfactants are chemical compounds that decrease the surface tension or interfacial tension between two liquids, a liquid and a gas, or a liquid and a solid. Surfactants may function as emulsifiers, wetting agents, detergents, foaming agents, or dispersants. The word "surfactant" is a blend of surface-active agent, coined c. 1950.
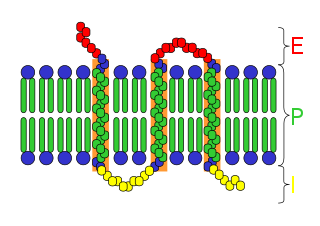
An integral, or intrinsic, membrane protein (IMP) is a type of membrane protein that is permanently attached to the biological membrane. All transmembrane proteins can be classified as IMPs, but not all IMPs are transmembrane proteins. IMPs comprise a significant fraction of the proteins encoded in an organism's genome. Proteins that cross the membrane are surrounded by annular lipids, which are defined as lipids that are in direct contact with a membrane protein. Such proteins can only be separated from the membranes by using detergents, nonpolar solvents, or sometimes denaturing agents.

A transmembrane protein is a type of integral membrane protein that spans the entirety of the cell membrane. Many transmembrane proteins function as gateways to permit the transport of specific substances across the membrane. They frequently undergo significant conformational changes to move a substance through the membrane. They are usually highly hydrophobic and aggregate and precipitate in water. They require detergents or nonpolar solvents for extraction, although some of them (beta-barrels) can be also extracted using denaturing agents.
A lysis buffer is a buffer solution used for the purpose of breaking open cells for use in molecular biology experiments that analyze the labile macromolecules of the cells. Most lysis buffers contain buffering salts and ionic salts to regulate the pH and osmolarity of the lysate. Sometimes detergents are added to break up membrane structures. For lysis buffers targeted at protein extraction, protease inhibitors are often included, and in difficult cases may be almost required. Lysis buffers can be used on both animal and plant tissue cells.
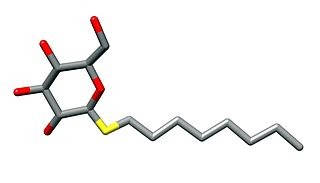
n-Octyl β-d-thioglucopyranoside is a mild nonionic detergent that is used for cell lysis or to solubilise membrane proteins without denaturing them. This is particularly of use in order to crystallise them or to reconstitute them into lipid bilayers. It has a critical micelle concentration of 9 mM.

Sodium triphosphate (STP), also sodium tripolyphosphate (STPP), or tripolyphosphate (TPP),) is an inorganic compound with formula Na5P3O10. It is the sodium salt of the polyphosphate penta-anion, which is the conjugate base of triphosphoric acid. It is produced on a large scale as a component of many domestic and industrial products, especially detergents. Environmental problems associated with eutrophication are attributed to its widespread use.
Ammonium sulfate precipitation is one of the most commonly used methods for large and laboratory scale protein purification and fractionation that can be used to separate proteins by altering their solubility in the presence of a high salt concentration.

An amphiphile, or amphipath, is a chemical compound possessing both hydrophilic and lipophilic (fat-loving) properties. Such a compound is called amphiphilic or amphipathic. Amphiphilic compounds include surfactants. The phospholipid amphiphiles are the major structural component of cell membranes.

Cetrimonium bromide, also known with the abbreviation CTAB, is a quaternary ammonium surfactant with a condensed structural formula [(C16H33)N(CH3)3]Br.
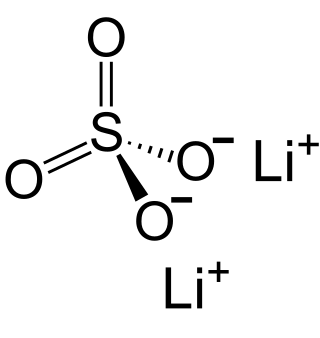
Lithium sulfate is a white inorganic salt with the formula Li2SO4. It is the lithium salt of sulfuric acid.

Surfactin is a cyclic lipopeptide, commonly used as an antibiotic for its capacity as a surfactant. It is an amphiphile capable of withstanding hydrophilic and hydrophobic environments. The Gram-positive bacterial species Bacillus subtilis produces surfactin for its antibiotic effects against competitors. Surfactin showcases antibacterial, antiviral, antifungal, and hemolytic effects.

Polymorphism in biophysics is the ability of lipids to aggregate in a variety of ways, giving rise to structures of different shapes, known as "phases". This can be in the form of spheres of lipid molecules (micelles), pairs of layers that face one another, a tubular arrangement (hexagonal), or various cubic phases. More complicated aggregations have also been observed, such as rhombohedral, tetragonal and orthorhombic phases.

Lyotropic liquid crystals result when fat-loving and water-loving chemical compounds known as amphiphiles dissolve into a solution that behaves both like a liquid and a solid crystal. This liquid crystalline mesophase includes everyday mixtures like soap and water.
Octyl glucoside is a nonionic surfactant frequently used to solubilise integral membrane proteins for studies in biochemistry. Structurally, it is a glycoside derived from glucose and octanol. Like Genapol X-100 and Triton X-100, it is a nonphysiological amphiphile that makes lipid bilayers less "stiff".
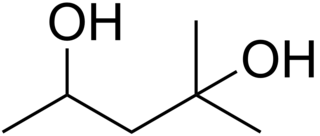
2-Methyl-2,4-pentanediol (MPD) is an organic compound with the formula (CH3)2C(OH)CH2CH(OH)CH3. This colourless liquid is a chiral diol. It is produced industrially from diacetone alcohol by hydrogenation. Total European and USA production was 15000 tonnes in 2000.

Protein crystallization is the process of formation of a regular array of individual protein molecules stabilized by crystal contacts. If the crystal is sufficiently ordered, it will diffract. Some proteins naturally form crystalline arrays, like aquaporin in the lens of the eye.
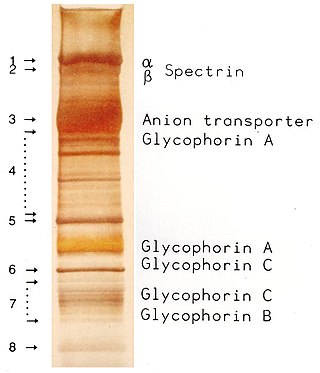
SDS-PAGE is a discontinuous electrophoretic system developed by Ulrich K. Laemmli which is commonly used as a method to separate proteins with molecular masses between 5 and 250 kDa. The combined use of sodium dodecyl sulfate and polyacrylamide gel eliminates the influence of structure and charge, and proteins are separated by differences in their size. At least up to 2012, the publication describing it was the most frequently cited paper by a single author, and the second most cited overall.
References
- ↑ Additives for protein crystallization and concentration range
- 1 2 Quantitative structure-activity relationship of human neutrophil collagenase (MMP-8) inhibitors using comparative molecular field analysis and X-ray structure analysis. doi : 10.2210/pdb1BZS/pdb
- ↑ Stura, E.A. (1998). "Strategy 3: Reverse Screening.". In Bergfors, T. (ed.). Crystallization of Proteins: Techniques, Strategies and Tips. A laboratory manual. International University Line. pp. 113–124.
- ↑ Stura E.A, Satterthwait, A.C, Calvo, J.C, Kaslow, D.C, Wilson, I.A. (1994). "Reverse Screening". Acta Crystallogr. D. 50 (4): 448–455. doi: 10.1107/S0907444994001794 . PMID 15299400.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Hartmut Michel, ed. (1991). Crystallization of membrane proteins. CRC Press.
- ↑ M Caffrey (2003). "Membrane protein crystallization". J. Struct. Biol. 142 (1): 108–132. doi:10.1016/S1047-8477(03)00043-1. PMID 12718924.
- ↑ "List of host lipids compatible with in meso crystallization trials". Archived from the original on 2011-12-02. Retrieved 2011-12-09.
- ↑ Misquitta Y.; Caffrey M. (2003). "Detergents Destabilize the Cubic Phase of Monoolein: Implications for Membrane Protein Crystallization". Biophys. J. 85 (5): 3084–3096. Bibcode:2003BpJ....85.3084M. doi:10.1016/S0006-3495(03)74727-4. PMC 1303585 . PMID 14581209.