
Amyloids are aggregates of proteins that become folded into a shape that allows many copies of that protein to stick together, forming fibrils. In the human body, amyloids have been linked to the development of various diseases. Pathogenic amyloids form when previously healthy proteins lose their normal physiological functions and form fibrous deposits in plaques around cells which can disrupt the healthy function of tissues and organs.

Ion chromatography is a chromatography process that separates ions and polar molecules based on their affinity to the ion exchanger. It works on almost any kind of charged molecule—including large proteins, small nucleotides, and amino acids. However, ion chromatography must be done in conditions that are one unit away from the isoelectric point of a protein.
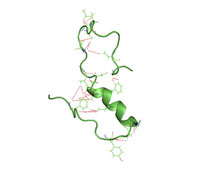
Amyloid beta denotes peptides of 36–43 amino acids that are crucially involved in Alzheimer's disease as the main component of the amyloid plaques found in the brains of Alzheimer patients. The peptides derive from the amyloid precursor protein (APP), which is cleaved by beta secretase and gamma secretase to yield Aβ. Aβ molecules can aggregate to form flexible soluble oligomers which may exist in several forms. It is now believed that certain misfolded oligomers can induce other Aβ molecules to also take the misfolded oligomeric form, leading to a chain reaction akin to a prion infection. The oligomers are toxic to nerve cells. The other protein implicated in Alzheimer's disease, tau protein, also forms such prion-like misfolded oligomers, and there is some evidence that misfolded Aβ can induce tau to misfold.
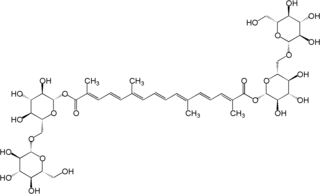
Crocin is a carotenoid chemical compound that is found in the flowers crocus and gardenia. Crocin is the chemical primarily responsible for the color of saffron.

Piceid is a stilbenoid glucoside and is a major resveratrol derivative in grape juices. It can be found in the bark of Picea sitchensis. It can also be isolated from Reynoutria japonica, the Japanese knotweed.

Gallocatechol or gallocatechin (GC) is a flavan-3-ol, a type of chemical compound including catechin, with the gallate residue being in an isomeric trans position. It is one of the antioxidant chemicals found in food.

Adrenosterone, also known as Reichstein's substance G , as well as 11-ketoandrostenedione (11-KA4), 11-oxoandrostenedione (11-OXO), and androst-4-ene-3,11,17-trione, is a steroid hormone with a weak androgenic effect, and an intermediate/prohormone of 11-ketotestosterone. It was first isolated in 1936 from the adrenal cortex by Tadeus Reichstein at the Pharmaceutical Institute in the University of Basel. Originally, adrenosterone was called Reichstein's substance G. Adrenosterone occurs in trace amounts in humans as well as most mammals and in larger amounts in fish, where it is a precursor to the primary androgen, 11-ketotestosterone.

Desformylflustrabromine (dFBr) is a tryptamine derivative which was first isolated as an active metabolite of the marine bryozoan Flustra foliacea.
Prodelphinidin is a name for the polymeric tannins composed of gallocatechin. It yields delphinidin during depolymerisation under oxidative conditions.

ε-Viniferin is a naturally occurring phenol, belonging to the stilbenoids family. It is a resveratrol dimer.

Chebulinic acid is an ellagitannin found in the seeds of Euphoria longana, in the fruits of Terminalia chebula or in the leaves of T. macroptera.
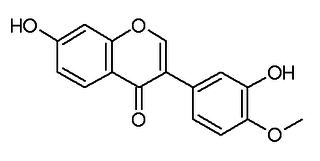
Calycosin is an O-methylated isoflavone. It can be isolated from Astragalus membranaceus Bge. var. mongholicus and Trifolium pratense L..

Countercurrent chromatography is a form of liquid–liquid chromatography that uses a liquid stationary phase that is held in place by centrifugal force and is used to separate, identify, and quantify the chemical components of a mixture. In its broadest sense, countercurrent chromatography encompasses a collection of related liquid chromatography techniques that employ two immiscible liquid phases without a solid support. The two liquid phases come in contact with each other as at least one phase is pumped through a column, a hollow tube or a series of chambers connected with channels, which contains both phases. The resulting dynamic mixing and settling action allows the components to be separated by their respective solubilities in the two phases. A wide variety of two-phase solvent systems consisting of at least two immiscible liquids may be employed to provide the proper selectivity for the desired separation.

Astringin is a stilbenoid, the 3-β-D-glucoside of piceatannol. It can be found in the bark of Picea sitchensis or Picea abies.
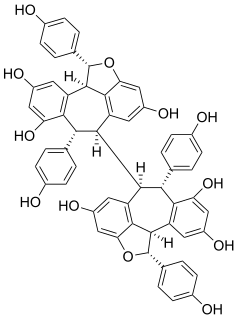
Hopeaphenol is a stilbenoid. It is a resveratrol tetramer. It has been first isolated from Dipterocarpaceae like Shorea ovalis. It has also been isolated from wines from North Africa.

Miquelianin is a flavonol glucuronide, a type of phenolic compound present in wine, in species of St John's wort, like Hypericum hirsutum, in Nelumbo nucifera or in green beans.

δ-Viniferin is a resveratrol dehydrodimer. It is an isomer of epsilon-viniferin. It can be isolated from stressed grapevine leaves. It is also found in plant cell cultures. or in wine. It can also be found in Rheum maximowiczii.
Droplet countercurrent chromatography was introduced in 1970 by Tanimura, Pisano, Ito, and Bowman. DCCC is considered to be a form of liquid-liquid separation, which includes countercurrent distribution and countercurrent chromatography, that employs a liquid stationary phase held in a collection of vertical glass columns connected in series. The mobile phase passes through the columns in the form of droplets. The DCCC apparatus may be run with the lower phase stationary and the upper phase being introduced to the bottom of each column. Or it may be run with the upper phase stationary and the lower phase being introduced from the top of the column. In both cases, the work of gravity is allowed influence the two immiscible liquids of different densities to form the signature droplets that rise or descend through the column. The mobile phase is pumped at a rate that will allow droplets to form that maximize the mass transfer of a compound between the upper and lower phases. Compounds that are more soluble in the upper phase will travel quickly through the column, while compounds that are more soluble in the stationary phase will linger. Separation occurs because different compounds distribute differently, in a ratio called the partition coefficient, between the two phases.



















