Satiety is a state or condition of fullness gratified beyond the point of satisfaction, the opposite of hunger. Following satiation, satiety is a feeling of fullness lasting until the next meal. When food is present in the GI tract after a meal, satiety signals overrule hunger signals, but satiety slowly fades as hunger increases.
Anti-obesity medication or weight loss medications are pharmacological agents that reduce or control excess body fat. These medications alter one of the fundamental processes of the human body, weight regulation, by: reducing appetite and consequently energy intake, increasing energy expenditure, redirecting nutrients from adipose to lean tissue, or interfering with the absorption of calories.

The glucagon-like peptide-1 receptor (GLP1R) is a G protein-coupled receptor (GPCR) found on beta cells of the pancreas and on neurons of the brain. It is involved in the control of blood sugar level by enhancing insulin secretion. In humans it is synthesised by the gene GLP1R, which is present on chromosome 6. It is a member of the glucagon receptor family of GPCRs. GLP1R is composed of two domains, one extracellular (ECD) that binds the C-terminal helix of GLP-1, and one transmembrane (TMD) domain that binds the N-terminal region of GLP-1. In the TMD domain there is a fulcrum of polar residues that regulates the biased signaling of the receptor while the transmembrane helical boundaries and extracellular surface are a trigger for biased agonism.
The orexin receptor (also referred to as the hypocretin receptor) is a G-protein-coupled receptor that binds the neuropeptide orexin. There are two variants, OX1 and OX2, each encoded by a different gene (HCRTR1, HCRTR2).

Orexin receptor type 2 (Ox2R or OX2), also known as hypocretin receptor type 2 (HcrtR2), is a protein that in humans is encoded by the HCRTR2 gene.

(−)-Stepholidine is a protoberberine alkaloid found in the plant Stephania intermedia.
Albiglutide is a glucagon-like peptide-1 agonist drug marketed by GlaxoSmithKline (GSK) for treatment of type 2 diabetes. As of 2017 it is unclear if it affects a person's risk of death. GSK has announced that it intends to withdraw the drug from the worldwide market by July 2018 for economic reasons.
Glucagon-like peptide-1 (GLP-1) receptor agonists, also known as GLP-1 analogs, GLP-1DAs or incretin mimetics, are a class of drugs that reduce blood sugar and energy intake by activating the GLP-1 receptor. They mimic the actions of the endogenous incretin hormone GLP-1 that is released by the gut after eating.
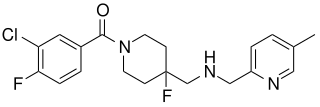
Befiradol is an experimental drug being studied for the treatment of levodopa-induced dyskinesia. It is a potent and selective 5-HT1A receptor full agonist.
Lixisenatide is a once-daily injectable GLP-1 receptor agonist for the treatment of type 2 diabetes.

Dulaglutide, sold under the brand name Trulicity among others, is a medication used for the treatment of type 2 diabetes in combination with diet and exercise. It is also approved in the United States for the reduction of major adverse cardiovascular events in adults with type 2 diabetes who have established cardiovascular disease or multiple cardiovascular risk factors. It is a once-weekly injection.
Semaglutide is an antidiabetic medication used for the treatment of type 2 diabetes and an anti-obesity medication used for long-term weight management. It was developed by Novo Nordisk in 2012 and approved for use in the US in 2017. It is a peptide similar to the hormone glucagon-like peptide-1 (GLP-1), modified with a side chain. It can be administered by subcutaneous injection or taken orally. It is sold under the brand names Ozempic (injectable) and Rybelsus (pill) for diabetes, and under the brand name Wegovy for weight loss.
Cotadutide is an experimental drug for the treatment of type 2 diabetes mellitus. It lowers blood glucose levels by mimicking the human hormones glucagon-like peptide 1 and glucagon, which play a role in blood sugar regulation. The drug is a peptide that is injected under the skin.

Tirzepatide, sold under the brand name Mounjaro among others, is an antidiabetic medication used for the treatment of type 2 diabetes and for weight loss. Tirzepatide is administered through subcutaneous injection.
Retatrutide (LY-3437943) is an experimental drug for obesity developed by American pharmaceutical company Eli Lilly and Company. It is a triple hormone receptor agonist of GLP-1, GIP, and GCGR receptors. It has been shown to achieve a more than 24% mean weight reduction in adults without diabetes but with obesity or preobesity (overweight) during a phase 2 trial. The drug is a peptide with amino acid sequence YA1QGTFTSDYSIL2LDKK4AQA1AFIEYLLEGGPSSGAPPPS3.
Mazdutide is a dual agonist of the GLP-1 receptor and glucagon receptor. It is an analog of oxyntomodulin (OXM). The drug is developed by Eli Lilly and is currently in a Phase II study.
GLP1 poly-agonist peptides are a class of drugs that activate multiple peptide hormone receptors including the glucagon-like peptide-1 (GLP-1) receptor. These drugs are developed for the same indications as GLP-1 receptor agonists—especially obesity, type 2 diabetes, and non-alcoholic fatty liver disease. They are expected to provide superior efficacy with fewer adverse effects compared to GLP-1 mono-agonists, which are dose-limited by gastrointestinal disturbances. The effectiveness of multi-receptor agonists could possibly equal or exceed that of bariatric surgery. The first such drug to receive approval is tirzepatide, a dual agonist of GLP-1 and GIP receptors.
HRS9531 is an experimental GLP-1 and GIPR dual agonist developed by Jiangsu Hengrui.
Survodutide is an experimental peptide that works as a dual glucagon/GLP-1 receptor agonist. Unlike other dual GLP-1/glucagon dual agonists, it is an glucagon analog rather than an analog of oxyntomodulin. It is developed by Boehringer Ingelheim as a weight loss drug.
Pemvidutide (ALT-801) is an experimental dual GLP-1/glucagon receptor agonist developed by Altimmune. The drug reduced LDL-C in a clinical trial and does not require dose titration as with GLP-1 mono agonists.






