Related Research Articles

Chronic lymphocytic leukemia (CLL) is a type of cancer in which the bone marrow makes too many lymphocytes. Early on, there are typically no symptoms. Later, non-painful lymph node swelling, feeling tired, fever, night sweats, or weight loss for no clear reason may occur. Enlargement of the spleen and low red blood cells (anemia) may also occur. It typically worsens gradually over years.

Rituximab, sold under the brand name Rituxan among others, is a monoclonal antibody medication used to treat certain autoimmune diseases and types of cancer. It is used for non-Hodgkin lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis, granulomatosis with polyangiitis, idiopathic thrombocytopenic purpura, pemphigus vulgaris, myasthenia gravis and Epstein–Barr virus-positive mucocutaneous ulcers. It is given by slow intravenous infusion. Biosimilars of Rituxan include Blitzima, Riabni, Ritemvia, Rituenza, Rixathon, Ruxience, and Truxima.

Cancer immunotherapy (immuno-oncotherapy) is the stimulation of the immune system to treat cancer, improving the immune system's natural ability to fight the disease. It is an application of the fundamental research of cancer immunology and a growing subspecialty of oncology.

Monoclonal gammopathy of undetermined significance (MGUS) is a plasma cell dyscrasia in which plasma cells or other types of antibody-producing cells secrete a myeloma protein, i.e. an abnormal antibody, into the blood; this abnormal protein is usually found during standard laboratory blood or urine tests. MGUS resembles multiple myeloma and similar diseases, but the levels of antibodies are lower, the number of plasma cells in the bone marrow is lower, and it rarely has symptoms or major problems. However, since MGUS can lead to multiple myeloma, which develops at the rate of about 1.5% a year, or other symptomatic conditions, yearly monitoring is recommended.

Ibritumomab tiuxetan, sold under the trade name Zevalin, is a monoclonal antibody radioimmunotherapy treatment for non-Hodgkin's lymphoma. The drug uses the monoclonal mouse IgG1 antibody ibritumomab in conjunction with the chelator tiuxetan, to which a radioactive isotope is added. Tiuxetan is a modified version of DTPA whose carbon backbone contains an isothiocyanatobenzyl and a methyl group.
Waldenström macroglobulinemia is a type of cancer affecting two types of B cells: lymphoplasmacytoid cells and plasma cells. Both cell types are white blood cells. It is characterized by having high levels of a circulating antibody, immunoglobulin M (IgM), which is made and secreted by the cells involved in the disease. Waldenström macroglobulinemia is an "indolent lymphoma" and a type of lymphoproliferative disease which shares clinical characteristics with the indolent non-Hodgkin lymphomas. It is commonly classified as a form of plasma cell dyscrasia, similar to other plasma cell dyscrasias that, for example, lead to multiple myeloma. Waldenström macroglobulinemia is commonly preceded by two clinically asymptomatic but progressively more pre-malignant phases, IgM monoclonal gammopathy of undetermined significance and smoldering Waldenström macroglobulinemia. The Waldenström macroglobulinemia spectrum of dysplasias differs from other spectrums of plasma cell dyscrasias in that it involves not only aberrant plasma cells but also aberrant lymphoplasmacytoid cells and that it involves IgM while other plasma dyscrasias involve other antibody isoforms.
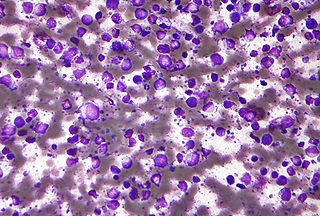
Diffuse large B-cell lymphoma (DLBCL) is a cancer of B cells, a type of lymphocyte that is responsible for producing antibodies. It is the most common form of non-Hodgkin lymphoma among adults, with an annual incidence of 7–8 cases per 100,000 people per year in the US and UK. This cancer occurs primarily in older individuals, with a median age of diagnosis at ~70 years, although it can occur in young adults and, in rare cases, children. DLBCL can arise in virtually any part of the body and, depending on various factors, is often a very aggressive malignancy. The first sign of this illness is typically the observation of a rapidly growing mass or tissue infiltration that is sometimes associated with systemic B symptoms, e.g. fever, weight loss, and night sweats.

Monoclonal antibodies (mAbs) have varied therapeutic uses. It is possible to create a mAb that binds specifically to almost any extracellular target, such as cell surface proteins and cytokines. They can be used to render their target ineffective, to induce a specific cell signal, to cause the immune system to attack specific cells, or to bring a drug to a specific cell type.
Richter's transformation (RT), also known as Richter's syndrome, is the conversion of chronic lymphocytic leukemia (CLL) or its variant, small lymphocytic lymphoma (SLL), into a new and more aggressively malignant disease. CLL is the circulation of malignant B lymphocytes with or without the infiltration of these cells into lymphatic or other tissues while SLL is the infiltration of these malignant B lymphocytes into lymphatic and/or other tissues with little or no circulation of these cells in the blood. CLL along with its SLL variant are grouped together in the term CLL/SLL.
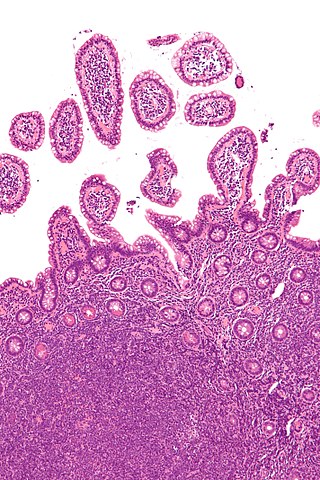
Mantle cell lymphoma (MCL) is a type of non-Hodgkin's lymphoma, comprising about 6% of cases. It is named for the mantle zone of the lymph nodes where it develops. The term 'mantle cell lymphoma' was first adopted by Raffeld and Jaffe in 1991.
Chemoimmunotherapy is chemotherapy combined with immunotherapy. Chemotherapy uses different drugs to kill or slow the growth of cancer cells; immunotherapy uses treatments to stimulate or restore the ability of the immune system to fight cancer. A common chemoimmunotherapy regimen is CHOP combined with rituximab (CHOP-R) for B-cell non-Hodgkin lymphomas.
Milatuzumab is an anti-CD74 humanized monoclonal antibody for the treatment of multiple myeloma non-Hodgkin's lymphoma and chronic lymphocytic leukemia.
Siltuximab is a chimeric monoclonal antibody. It binds to interleukin-6. Siltuximab has been investigated for the treatment of neoplastic diseases: metastatic renal cell cancer, prostate cancer, other types of cancer, and for Castleman's disease.

Marginal zone B-cell lymphomas, also known as marginal zone lymphomas (MZLs), are a heterogeneous group of lymphomas that derive from the malignant transformation of marginal zone B-cells. Marginal zone B cells are innate lymphoid cells that normally function by rapidly mounting IgM antibody immune responses to antigens such as those presented by infectious agents and damaged tissues. They are lymphocytes of the B-cell line that originate and mature in secondary lymphoid follicles and then move to the marginal zones of mucosa-associated lymphoid tissue, the spleen, or lymph nodes. Mucosa-associated lymphoid tissue is a diffuse system of small concentrations of lymphoid tissue found in various submucosal membrane sites of the body such as the gastrointestinal tract, mouth, nasal cavity, pharynx, thyroid gland, breast, lung, salivary glands, eye, skin and the human spleen.

Nodular lymphocyte predominant Hodgkin lymphoma (NLPHL) is a slow-growing CD20 positive form of Hodgkin lymphoma, a cancer of the immune system's B cells.

Two chemically linked fragments antigen-binding form an artificial antibody that binds to two different antigens, making it a type of bispecific antibody. They are fragments antigen-binding of two different monoclonal antibodies and are linked by chemical means like a thioether. Typically, one of the Fabs binds to a tumour antigen and the other to a protein on the surface of an immune cell, for example an Fc receptor on a macrophage. In this way, tumour cells are attached to immune cells, which destroy them.
Gene expression profiling has revealed that diffuse large B-cell lymphoma (DLBCL) is composed of at least 3 different sub-groups, each having distinct oncogenic mechanisms that respond to therapies in different ways. Germinal Center B-Cell like (GCB) DLBCLs appear to arise from normal germinal center B cells, while Activated B-cell like (ABC) DLBCLs are thought to arise from postgerminal center B cells that are arrested during plasmacytic differentiation. The differences in gene expression between GCB DLBCL and ABC DLBCL are as vast as the differences between distinct types of leukemia, but these conditions have historically been grouped together and treated as the same disease.

Daratumumab, sold under the brand name Darzalex, is an anti-cancer monoclonal antibody medication. It binds to CD38, which is overexpressed in multiple myeloma cells. Daratumumab was originally developed by Genmab, but it is now being jointly developed by Genmab along with the Johnson & Johnson subsidiary Janssen Biotech, which acquired worldwide commercialization rights to the drug from Genmab.
The NK-92 cell line is an immortalised cell line that has the characteristics of a type of immune cell found in human blood called ’natural killer’ (NK) cells. Blood NK cells and NK-92 cells recognize and attack cancer cells as well as cells that have been infected with a virus, bacteria, or fungus. NK-92 cells were first isolated in 1992 in the laboratory of Hans Klingemann at the British Columbia Cancer Agency in Vancouver, Canada, from a patient who had a rare NK cell non-Hodgkin-lymphoma. These cells were subsequently developed into a continuously growing cell line. NK-92 cells are distinguished by their suitability for expansion to large numbers, ability to consistently kill cancer cells and testing in clinical trials. When NK-92 cells recognize a cancerous or infected cell, they secrete perforin that opens holes into the diseased cells and releases granzymes that kill the target cells. NK-92 cells are also capable of producing cytokines such as tumor necrosis factor alpha (TNF-a) and interferon gamma (IFN-y), which stimulates proliferation and activation of other immune cells.
Passive antibody therapy, also called serum therapy, is a subtype of passive immunotherapy that administers antibodies to target and kill pathogens or cancer cells. It is designed to draw support from foreign antibodies that are donated from a person, extracted from animals, or made in the laboratory to elicit an immune response instead of relying on the innate immune system to fight disease. It has a long history from the 18th century for treating infectious diseases and is now a common cancer treatment. The mechanism of actions include: antagonistic and agonistic reaction, complement-dependent cytotoxicity (CDC), and antibody-dependent cellular cytotoxicity (ADCC).
References
- ↑ McGinley, Laurie (November 1, 2016). "Seattle center bets big on experimental immunotherapy". Washington Post.
- 1 2 3 "Whitworth Alumni - Alumni Award Winners & Alumni of Note". connect.whitworth.edu. Retrieved November 1, 2016.
- 1 2 3 "David G. Maloney M.D.. Ph.D." UW Medicine. Retrieved November 1, 2016.
- ↑ "Stanford Cancer Expert Ronald Levy Will Receive King Faisal Prize in Medicine March 29 | Business Wire". www.businesswire.com (Press release). Retrieved November 1, 2016.
- ↑ "Maloney DG[Author] - PubMed - NCBI". www.ncbi.nlm.nih.gov. Retrieved November 1, 2016.
- ↑ Lynch, Richard (March 4, 1982). "Immunoregulation of Malignant Lymphoma". New England Journal of Medicine. 306 (9): 543–544. doi:10.1056/NEJM198203043060912. ISSN 0028-4793. PMID 6977092.
- 1 2 Downey, Philip (July 20, 2010). "Profile of Ronald Levy". Proceedings of the National Academy of Sciences. 107 (29): 12745–12746. Bibcode:2010PNAS..10712745A. doi: 10.1073/pnas.1008810107 . ISSN 0027-8424. PMC 2919931 . PMID 20624971.
- ↑ Waldmann, Thomas A.; Levy, Ronald; Coller, Barry S. (January 1, 2000). "Emerging Therapies: Spectrum of Applications of Monoclonal Antibody Therapy". ASH Education Program Book. 2000 (1): 394–408. doi:10.1182/asheducation-2000.1.394. ISSN 1520-4391. PMID 11701553. S2CID 23849896.
- ↑ Cheson, Bruce D.; Leonard, John P. (August 7, 2008). "Monoclonal Antibody Therapy for B-Cell Non-Hodgkin's Lymphoma". New England Journal of Medicine. 359 (6): 613–626. doi:10.1056/NEJMra0708875. ISSN 0028-4793. PMID 18687642.
- ↑ Leukemia & Lymphoma Society (September 1, 2015). "Our History". www.lls.org. Archived from the original on October 8, 2018. Retrieved November 1, 2016.
- ↑ Horning, Sandra J.; Mocharnuk, Robert S. (February 27, 2002). "Monoclonal Antibody Therapy in B-Cell-Related Disorders and Malignancies". www.medscape.org. Retrieved November 1, 2016.
- ↑ Peck, Peggy (December 9, 2001). "Dual bone marrow transplants aid myeloma". UPI. Retrieved November 1, 2016.
- ↑ Vesole, David H. (November 1, 2003). ""Full length, midi, or mini": a fashion statement for transplants in myeloma". Blood. 102 (9): 3081–3082. doi: 10.1182/blood-2003-08-2910 . ISSN 0006-4971.
- ↑ Park, Alice (March 24, 2016). "What If Your Immune System Could Be Taught to Kill Cancer?". Time . Retrieved November 1, 2016.
- ↑ Helwick, Caroline (August 10, 2016). "CAR T-Cell Therapy Promising in Non-Hodgkin Lymphoma - The ASCO Post". www.ascopost.com. Retrieved November 1, 2016.
- ↑ Aleccia, JoNel (April 25, 2016). "New twist on T-cell therapy puts leukemia patients in remission". The Seattle Times . Retrieved November 1, 2016.
- ↑ Dillman, Robert O. (2005). "International Society for Biological Therapy of Cancer: 20th Anniversary". Journal of Immunotherapy. 28 (3): 169–174. doi:10.1097/01.cji.0000162778.11558.5d. PMID 15838372.
- ↑ "David G Maloney, MD, PHD - Medical Oncologist in Seattle, WA - Vitals.com". Vitals. Retrieved November 1, 2016.
- ↑ "September 2016: Remembering Alfred G. Knudson Jr., MD, PhD and James G. White, MD, ASH Bridge Grant awards, and more - ASH Clinical News". ASH Clinical News. September 1, 2016. Archived from the original on November 17, 2016. Retrieved November 1, 2016.