David W. Murhammer is professor and former department chair of chemical and biochemical engineering at the University of Iowa, specializing in biochemical engineering. He is also a member of the Center for Biocatalysis and Bioprocessing there. Murhammer received his B.S. and M.S. degrees from Oregon State University, and his Ph.D. in chemical engineering at the University of Houston in 1989. Murhammer was a graduate student under Charles F. Goochee. He then joined the college of engineering in 1989 as assistant professor, becoming professor in 2003.
Murhammer also worked briefly in the Zirconium refining industry, which sparked his lifelong dedication to chemical process safety.
Research interests include baculoviruses, with emphasis on their bioinsecticide production and effects on insect cell cultures.
Murhammer is an editor of Baculovirus and Insect Cell Expression Protocols. He has also made numerous presentations at government and industrial engineering laboratories and conferences. He has written a total of 43 journal articles. Among his major recent peer-reviewed publications are:
He is Associate Editor of Applied Biochemistry and Biotechnology, and a member of the American Chemical Society; American Institute of Chemical Engineers; American Society for Engineering Education; Society for Free Radical Biology and Medicine.
He regularly teaches courses in "Process calculations", "Engineering flow/Heat exchange", "Chemical process safety", and "Introduction to biochemical engineering".
Antioxidants are compounds that inhibit oxidation, a chemical reaction that can produce free radicals. Autoxidation leads to degradation of organic compounds, including living matter. Antioxidants are frequently added to industrial products, such as polymers, fuels, and lubricants, to extend their useable lifetimes. Food are also treated with antioxidants to forestall spoilage, in particular the rancidification of oils and fats. In cells, antioxidants such as glutathione, mycothiol or bacillithiol, and enzyme systems like superoxide dismutase, can prevent damage from oxidative stress.
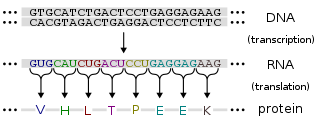
Protein production is the biotechnological process of generating a specific protein. It is typically achieved by the manipulation of gene expression in an organism such that it expresses large amounts of a recombinant gene. This includes the transcription of the recombinant DNA to messenger RNA (mRNA), the translation of mRNA into polypeptide chains, which are ultimately folded into functional proteins and may be targeted to specific subcellular or extracellular locations.
Fenton's reagent is a solution of hydrogen peroxide (H2O2) with ferrous iron (typically iron(II) sulfate, FeSO4) as a catalyst that is used to oxidize contaminants or waste waters as part of an advanced oxidation process. Fenton's reagent can be used to destroy organic compounds such as trichloroethylene (TCE) and tetrachloroethylene (perchloroethylene, PCE). It was developed in the 1890s by Henry John Horstman Fenton as an analytical reagent.

Baculoviridae is a family of viruses. Arthropods, among the most studied being Lepidoptera, Hymenoptera and Diptera, serve as natural hosts. Currently, 85 species are placed in this family, assigned to four genera.

Oxidative stress reflects an imbalance between the systemic manifestation of reactive oxygen species and a biological system's ability to readily detoxify the reactive intermediates or to repair the resulting damage. Disturbances in the normal redox state of cells can cause toxic effects through the production of peroxides and free radicals that damage all components of the cell, including proteins, lipids, and DNA. Oxidative stress from oxidative metabolism causes base damage, as well as strand breaks in DNA. Base damage is mostly indirect and caused by the reactive oxygen species generated, e.g., O2− (superoxide radical), OH (hydroxyl radical) and H2O2 (hydrogen peroxide). Further, some reactive oxidative species act as cellular messengers in redox signaling. Thus, oxidative stress can cause disruptions in normal mechanisms of cellular signaling.
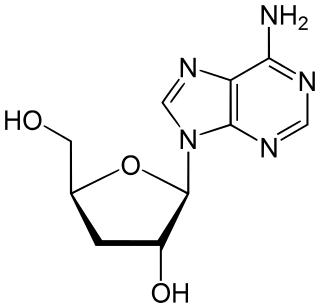
Cordycepin, or 3'-deoxyadenosine, is a derivative of the nucleoside adenosine, differing from the latter by the replacement of the hydroxy group in the 3' position with a hydrogen. It was initially extracted from the fungus Cordyceps militaris, but can now be produced synthetically. It is also found in other Cordyceps species as well as Ophiocordyceps sinensis.
NAD+ kinase (EC 2.7.1.23, NADK) is an enzyme that converts nicotinamide adenine dinucleotide (NAD+) into NADP+ through phosphorylating the NAD+ coenzyme. NADP+ is an essential coenzyme that is reduced to NADPH primarily by the pentose phosphate pathway to provide reducing power in biosynthetic processes such as fatty acid biosynthesis and nucleotide synthesis. The structure of the NADK from the archaean Archaeoglobus fulgidus has been determined.

Irwin Fridovich was an American biochemist who, together with his graduate student Joe M. McCord, discovered the enzymatic activity of copper-zinc superoxide dismutase (SOD),—to protect organisms from the toxic effects of superoxide free radicals formed as a byproduct of normal oxygen metabolism. Subsequently, Fridovich's research group also discovered the manganese-containing and the iron-containing SODs from Escherichia coli and the mitochondrial MnSOD (SOD2), now known to be an essential protein in mammals. He spent the rest of his career studying the biochemical mechanisms of SOD and of biological superoxide toxicity, using bacteria as model systems. Fridovich was also Professor Emeritus of Biochemistry at Duke University.

Extracellular superoxide dismutase [Cu-Zn] is an enzyme that in humans is encoded by the SOD3 gene.

Aldehyde dehydrogenase, dimeric NADP-preferring is an enzyme that in humans is encoded by the ALDH3A1 gene.

γ-L-Glutamyl-L-cysteine, also known as γ-glutamylcysteine (GGC), is a dipeptide found in animals, plants, fungi, some bacteria, and archaea. It has a relatively unusual γ-bond between the constituent amino acids, L-glutamic acid and L-cysteine and is a key intermediate in the γ-glutamyl cycle first described by Meister in the 1970s. It is the most immediate precursor to the antioxidant glutathione.

Nitrotyrosine is a product of tyrosine nitration mediated by reactive nitrogen species such as peroxynitrite anion and nitrogen dioxide. Nitrotyrosine is identified as an indicator or marker of cell damage, inflammation as well as NO (nitric oxide) production. Nitrotyrosine is formed in the presence of the active metabolite NO. Generally in many disease states, oxidative stress increases the production of superoxide (O2−) and NO forming peroxynitrite (ONOO−) a destructive free radical oxidant. The production of ONOO− is capable of oxidizing several lipoproteins and of nitrating tyrosine residues in many proteins. It is difficult to determine the production of ONOO− so, usually nitrotyrosine in proteins are the detectable marker for indirectly detecting ONOO−. It is detected in large number of pathological conditions and is considered a marker of NO-dependent, reactive nitrogen species-induced nitrative stress. Nitrotyrosine is detected in biological fluids such as plasma, lung aspirants-BALF (Broncho alveolar lining fluid) and urine. Increased level of nitrotyrosine is detected in rheumatoid arthritis, septic shock and coeliac disease. In all these studies nitrotyrosine was undetected in healthy subjects. Nitrotyrosine is also found in numerous other disease-affected tissues, such as the cornea in keratoconus. Peroxynitrite and/or nitrative stress may participate in the pathogenesis of diabetes.
Baculovirus gene transfer into Mammalian cells, known from scientific research articles as BacMam, is the use of baculovirus to deliver genes to mammalian cells. Baculoviruses are insect cell viruses that can be modified to express proteins in mammalian cells. The unmodified baculovirus is able to enter those cells; however, its genes are not expressed unless a mammalian recognizable promoter is incorporated upstream of a gene of interest. Both unmodified baculovirus and its modified counterpart are unable to replicate in humans and are thus non-infectious.
High Five (BTI-Tn-5B1-4) is an insect cell line that originated from the ovarian cells of the cabbage looper, Trichoplusia ni. It was developed by the Boyce Thompson Institute for Plant Research.
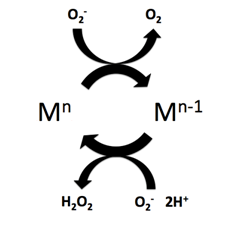
Superoxide dismutase (SOD) mimetics are synthetic compounds that mimic the native superoxide dismutase enzyme. SOD mimetics effectively convert the superoxide anion, a reactive oxygen species, into hydrogen peroxide, which is further converted into water by catalase. Reactive oxygen species are natural byproducts of cellular respiration and cause oxidative stress and cell damage, which has been linked to causing cancers, neurodegeneration, age-related declines in health, and inflammatory diseases. SOD mimetics are a prime interest in therapeutic treatment of oxidative stress because of their smaller size, longer half-life, and similarity in function to the native enzyme.
Katrina Miranda is an associate professor of biochemistry at the University of Arizona. She works on nitric oxide and their role in diseases like breast cancer, stroke and chronic pain.
Helicoverpa zea nudivirus 2 is an enveloped, rod-shaped, nonoccluded, double stranded DNA (dsDNA) sexually transmitted virus whose natural host is the corn earworm moth. At about 440 by 90 nm, it is the causative agent of the only sexually transmitted viral disease of any insect. It was originally identified in a colony of corn earworm moths established and maintained in Stoneville, Mississippi, U.S. and was found to be responsible for the sterility of those infected.

Reactive aldehyde species (RASP), also known as reactive aldehydes, refer to a class of electrophilic organic aldehyde molecules that are generally toxic or facilitate inflammation. RASP covalently react with amine groups and thiol groups, particularly in proteins. Following threshold amounts of binding to the electrophile-responsive proteome, RASP modify protein function, as has been described with MAP kinase, protein kinase C, and other proteins that potentiate cytokine release and other aspects of inflammation. Binding of RASP to proteins can also lead to NF-kB activation, autoantibody formation, inflammasome activation, and activation of Scavenger Receptor A. RASP are formed via a variety of processes, including oxidation of alcohols, polyamine metabolism and lipid peroxidation. In addition to binding to proteins and other amine or thiol-containing molecules such as glutathione, RASP are metabolized by aldehyde dehydrogenases or aldehyde reductases. Due to the toxicity of RASP, only a small number of genetic mutations in aldehyde dehydrogenases allow for viable offspring, resulting in Sjögren-Larsson Syndrome, Succinic Semi-Aldehyde Dehydrogenase Deficiency, and other rare diseases.

Helmut Sies is a German physician, biochemist and university professor. He was the first to demonstrate the existence of hydrogen peroxide as a normal attribute of aerobic life in 1970, and he introduced the concept of Oxidative stress in 1985. He also worked on the biological strategies of antioxidant defense and the biochemistry of nutritional antioxidants.

J. Paul Robinson is an Australian/American educator, biologist, biomedical engineer, and expert in the applications of flow cytometry. He is a Distinguished Professor of Cytometry in the Purdue University College of Veterinary Medicine, Department of Basic Medical Sciences, a Professor of Biomedical Engineering in the Weldon School of Biomedical Engineering, a Professor of Computer and Information Management at Purdue University, an Adjunct Professor of Microbiology & Immunology at West Lafayette Center for Medical Education, Indiana University School of Medicine, and the Director of Purdue University Cytometry Laboratories.