
Apoptosis is a form of programmed cell death that occurs in multicellular organisms. Biochemical events lead to characteristic cell changes (morphology) and death. These changes include blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation, DNA fragmentation, and mRNA decay. The average adult human loses between 50 and 70 billion cells each day due to apoptosis. For an average human child between eight and fourteen years old, approximately twenty to thirty billion cells die per day.

Cytokines are a broad and loose category of small proteins important in cell signaling. Cytokines are peptides and cannot cross the lipid bilayer of cells to enter the cytoplasm. Cytokines have been shown to be involved in autocrine, paracrine and endocrine signaling as immunomodulating agents.

Tumor necrosis factor is an adipokine and a cytokine. TNF is a member of the TNF superfamily, which consists of various transmembrane proteins with a homologous TNF domain.

In the field of cell biology, TNF-related apoptosis-inducing ligand (TRAIL), is a protein functioning as a ligand that induces the process of cell death called apoptosis.

The tumor necrosis factor receptor superfamily (TNFRSF) is a protein superfamily of cytokine receptors characterized by the ability to bind tumor necrosis factors (TNFs) via an extracellular cysteine-rich domain. With the exception of nerve growth factor (NGF), all TNFs are homologous to the archetypal TNF-alpha. In their active form, the majority of TNF receptors form trimeric complexes in the plasma membrane. Accordingly, most TNF receptors contain transmembrane domains (TMDs), although some can be cleaved into soluble forms, and some lack a TMD entirely. In addition, most TNF receptors require specific adaptor protein such as TRADD, TRAF, RIP and FADD for downstream signalling. TNF receptors are primarily involved in apoptosis and inflammation, but they can also take part in other signal transduction pathways, such as proliferation, survival, and differentiation. TNF receptors are expressed in a wide variety of tissues in mammals, especially in leukocytes.

Tumor necrosis factor receptor superfamily, member 4 (TNFRSF4), also known as CD134 and OX40 receptor, is a member of the TNFR-superfamily of receptors which is not constitutively expressed on resting naïve T cells, unlike CD28. OX40 is a secondary co-stimulatory immune checkpoint molecule, expressed after 24 to 72 hours following activation; its ligand, OX40L, is also not expressed on resting antigen presenting cells, but is following their activation. Expression of OX40 is dependent on full activation of the T cell; without CD28, expression of OX40 is delayed and of fourfold lower levels.

Caspase-8 is a caspase protein, encoded by the CASP8 gene. It most likely acts upon caspase-3. CASP8 orthologs have been identified in numerous mammals for which complete genome data are available. These unique orthologs are also present in birds.

TNF receptor-associated factor 2 is a protein that in humans is encoded by the TRAF2 gene.
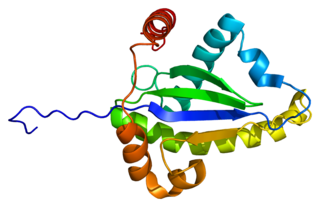
Tumor necrosis factor receptor type 1-associated DEATH domain protein is a protein that in humans is encoded by the TRADD gene.

Lymphotoxin beta receptor (LTBR), also known as tumor necrosis factor receptor superfamily member 3 (TNFRSF3), is a cell surface receptor for lymphotoxin involved in apoptosis and cytokine release. It is a member of the tumor necrosis factor receptor superfamily.

Tumor necrosis factor receptor 1 (TNFR1), also known as tumor necrosis factor receptor superfamily member 1A (TNFRSF1A) and CD120a, is a ubiquitous membrane receptor that binds tumor necrosis factor-alpha (TNFα).

TNF receptor-associated factor 1 is a protein that in humans is encoded by the TRAF1 gene.

TNF receptor-associated factor 5 is a protein that in humans is encoded by the TRAF5 gene.

TNF receptor-associated factor (TRAF3) is a protein that in humans is encoded by the TRAF3 gene.

Receptor-interacting serine/threonine-protein kinase 1 (RIPK1) functions in a variety of cellular pathways related to both cell survival and death. In terms of cell death, RIPK1 plays a role in apoptosis and necroptosis. Some of the cell survival pathways RIPK1 participates in include NF-κB, Akt, and JNK.

Tumor necrosis factor, alpha-induced protein 3 or A20 is a protein that in humans is encoded by the TNFAIP3 gene.

Mitogen-activated protein kinase kinase kinase 14 also known as NF-kappa-B-inducing kinase (NIK) is an enzyme that in humans is encoded by the MAP3K14 gene.

Vascular endothelial growth inhibitor (VEGI), also known as TNF-like ligand 1A (TL1A) and TNF superfamily member 15 (TNFSF15), is protein that in humans is encoded by the TNFSF15 gene. VEGI is an anti-angiogenic protein. It belongs to tumor necrosis factor (ligand) superfamily, where it is member 15. It is the sole known ligand for death receptor 3, and it can also be recognized by decoy receptor 3.

Tumor necrosis factor receptor superfamily member 18 (TNFRSF18), also known as glucocorticoid-induced TNFR-related protein (GITR) or CD357. GITR is encoded and tnfrsf18 gene at chromosome 4 in mice. GITR is type I transmembrane protein and is described in 4 different isoforms. GITR human orthologue, also called activation-inducible TNFR family receptor (AITR), is encoded by the TNFRSF18 gene at chromosome 1.

Tumor necrosis factor receptor 2 (TNFR2), also known as tumor necrosis factor receptor superfamily member 1B (TNFRSF1B) and CD120b, is one of two membrane receptors that binds tumor necrosis factor-alpha (TNFα). Like its counterpart, tumor necrosis factor receptor 1 (TNFR1), the extracellular region of TNFR2 consists of four cysteine-rich domains which allow for binding to TNFα. TNFR1 and TNFR2 possess different functions when bound to TNFα due to differences in their intracellular structures, such as TNFR2 lacking a death domain (DD).


















