
Progressive multifocal leukoencephalopathy (PML) is a rare and often fatal viral disease characterized by progressive damage (-pathy) or inflammation of the white matter (leuko-) of the brain (-encephalo-) at multiple locations (multifocal). It is caused by the JC virus, which is normally present and kept under control by the immune system. The JC virus is harmless except in cases of weakened immune systems. In general, PML has a mortality rate of 30–50% in the first few months, and those who survive can be left with varying degrees of neurological disabilities.
Immunosuppression is a reduction of the activation or efficacy of the immune system. Some portions of the immune system itself have immunosuppressive effects on other parts of the immune system, and immunosuppression may occur as an adverse reaction to treatment of other conditions.

Mycophenolic acid is an immunosuppressant medication used to prevent rejection following organ transplantation and to treat autoimmune conditions such as Crohn's disease and lupus. Specifically it is used following kidney, heart, and liver transplantation. It can be given by mouth or by injection into a vein. It comes as mycophenolate sodium and mycophenolate mofetil.

Polyomaviridae is a family of viruses whose natural hosts are primarily mammals and birds. As of 2020, there are six recognized genera and 117 species, five of which are unassigned to a genus. 14 species are known to infect humans, while others, such as Simian Virus 40, have been identified in humans to a lesser extent. Most of these viruses are very common and typically asymptomatic in most human populations studied. BK virus is associated with nephropathy in renal transplant and non-renal solid organ transplant patients, JC virus with progressive multifocal leukoencephalopathy, and Merkel cell virus with Merkel cell cancer.

Human polyomavirus 2, commonly referred to as the JC virus or John Cunningham virus, is a type of human polyomavirus. It was identified by electron microscopy in 1965 by ZuRhein and Chou, and by Silverman and Rubinstein, and later isolated in culture and named using the two initials of a patient, John Cunningham, with progressive multifocal leukoencephalopathy (PML). The virus causes PML and other diseases only in cases of immunodeficiency, as in AIDS or during treatment with immunosuppressive drugs.
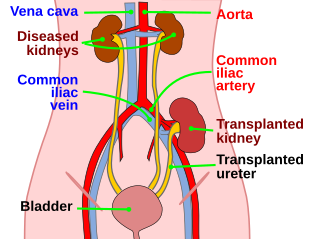
Kidney transplant or renal transplant is the organ transplant of a kidney into a patient with end-stage kidney disease (ESRD). Kidney transplant is typically classified as deceased-donor or living-donor transplantation depending on the source of the donor organ. Living-donor kidney transplants are further characterized as genetically related (living-related) or non-related (living-unrelated) transplants, depending on whether a biological relationship exists between the donor and recipient.

Ganciclovir, sold under the brand name Cytovene among others, is an antiviral medication used to treat cytomegalovirus (CMV) infections.

The BK virus is a member of the polyomavirus family. Past infection with the BK virus is widespread, but significant consequences of infection are uncommon, with the exception of the immunocompromised and the immunosuppressed. BK virus is an abbreviation of the name of the first patient, from whom the virus was isolated in 1971.

Leflunomide, sold under the brand name Arava among others, is an immunosuppressive disease-modifying antirheumatic drug (DMARD), used in active moderate-to-severe rheumatoid arthritis and psoriatic arthritis. It is a pyrimidine synthesis inhibitor that works by inhibiting dihydroorotate dehydrogenase.

Renal papillary necrosis is a form of nephropathy involving the necrosis of the renal papilla. Lesions that characterize renal papillary necrosis come from an impairment of the blood supply and from subsequent ischemic necrosis that is diffuse.
Human betaherpesvirus 5, also called human cytomegalovirus (HCMV), is species of virus in the genus Cytomegalovirus, which in turn is a member of the viral family known as Herpesviridae or herpesviruses. It is also commonly called CMV. Within Herpesviridae, HCMV belongs to the Betaherpesvirinae subfamily, which also includes cytomegaloviruses from other mammals. CMV is a double-stranded DNA virus.
Merkel cell polyomavirus was first described in January 2008 in Pittsburgh, Pennsylvania. It was the first example of a human viral pathogen discovered using unbiased metagenomic next-generation sequencing with a technique called digital transcriptome subtraction. MCV is one of seven currently known human oncoviruses. It is suspected to cause the majority of cases of Merkel cell carcinoma, a rare but aggressive form of skin cancer. Approximately 80% of Merkel cell carcinoma (MCC) tumors have been found to be infected with MCV. MCV appears to be a common—if not universal—infection of older children and adults. It is found in respiratory secretions suggesting that it may be transmitted by a respiratory route. But it also can be found shedding from healthy skin, and in gastrointestinal tract tissues and elsewhere, and so its precise mode of transmission remains unknown. In addition, recent studies suggest that this virus may latently infect the human sera and peripheral blood mononuclear cells.
Cytomegalic inclusion body disease (CIBD) also known as cytomegalic inclusion disease (CID) is a series of signs and symptoms caused by cytomegalovirus infection, toxoplasmosis or other rare infections such as herpes or rubella viruses. It can produce massive calcification of the central nervous system, and often the kidneys.
A Cytomegalovirus vaccine is a vaccine to prevent cytomegalovirus (CMV) infection or curb virus re-activation in persons already infected. Challenges in developing a vaccine include adeptness of CMV in evading the immune system and limited animal models. As of 2018 no such vaccine exists, although a number of vaccine candidates are under investigation. They include recombinant protein, live attenuated, DNA and other vaccines.

Trichodysplasia spinulosa is a rare cutaneous condition that has been described almost exclusively in immunocompromised patients, usually organ transplant recipients, on regimens of immunosuppressive drugs. As of early 2016, a total of 32 cases had been reported in the medical literature. Despite its rarity, TS is believed to be underdiagnosed, and the growing population of patients on immunosuppressive drug regimens suggests its incidence may rise. TS has been described as an emerging infectious disease.
WU polyomavirus is a virus of the family Polyomaviridae. It was discovered in 2007 in samples of human respiratory secretions, originally from a child patient in Australia who presented with clinical signs of pneumonia and in whom other common respiratory viruses were not detected. Follow-up studies identified the presence of WU virus in respiratory secretion samples from patients in Australia and the United States, suggesting that, like other human polyomaviruses, WU virus is widely distributed.
Human polyomavirus 7 (HPyV7) is a virus of the polyomavirus family that infects human hosts. It was discovered in 2010 and is a common component of the skin flora in healthy adults. There is limited evidence from case reports linking the virus to a skin rash occurring in immunocompromised organ transplant recipients.
Human polyomavirus 6 (HPyV6) is a virus of the polyomavirus family that infects human hosts. It was discovered in 2010 and is a component of the skin flora in healthy adults.
MW polyomavirus is a virus of the polyomavirus family that infects human hosts. It was discovered in 2012 and reported independently by several research groups. It has been identified mostly in stool samples from children and has been detected in a variety of geographic locations.
New Jersey polyomavirus is a virus of the polyomavirus family that infects human hosts. It was first identified in 2014 in a pancreatic transplant patient in New Jersey. It is the 13th and most recent human polyomavirus to be described.












