
Anticoagulants, commonly known as blood thinners, are chemical substances that prevent or reduce coagulation of blood, prolonging the clotting time. Some of them occur naturally in blood-eating animals such as leeches and mosquitoes, where they help keep the bite area unclotted long enough for the animal to obtain some blood. As a class of medications, anticoagulants are used in therapy for thrombotic disorders. Oral anticoagulants (OACs) are taken by many people in pill or tablet form, and various intravenous anticoagulant dosage forms are used in hospitals. Some anticoagulants are used in medical equipment, such as sample tubes, blood transfusion bags, heart-lung machines, and dialysis equipment. One of the first anticoagulants, warfarin, was initially approved as a rodenticide.

A thrombus, colloquially called a blood clot, is the final product of the blood coagulation step in hemostasis. There are two components to a thrombus: aggregated platelets and red blood cells that form a plug, and a mesh of cross-linked fibrin protein. The substance making up a thrombus is sometimes called cruor. A thrombus is a healthy response to injury intended to stop and prevent further bleeding, but can be harmful in thrombosis, when a clot obstructs blood flow through healthy blood vessels in the circulatory system.

Coagulation, also known as clotting, is the process by which blood changes from a liquid to a gel, forming a blood clot. It potentially results in hemostasis, the cessation of blood loss from a damaged vessel, followed by repair. The mechanism of coagulation involves activation, adhesion and aggregation of platelets, as well as deposition and maturation of fibrin.

Disseminated intravascular coagulation (DIC) is a condition in which blood clots form throughout the body, blocking small blood vessels. Symptoms may include chest pain, shortness of breath, leg pain, problems speaking, or problems moving parts of the body. As clotting factors and platelets are used up, bleeding may occur. This may include blood in the urine, blood in the stool, or bleeding into the skin. Complications may include organ failure.

Fibrin is a fibrous, non-globular protein involved in the clotting of blood. It is formed by the action of the protease thrombin on fibrinogen, which causes it to polymerize. The polymerized fibrin, together with platelets, forms a hemostatic plug or clot over a wound site.

Fibrinogen is a glycoprotein complex, produced in the liver, that circulates in the blood of all vertebrates. During tissue and vascular injury, it is converted enzymatically by thrombin to fibrin and then to a fibrin-based blood clot. Fibrin clots function primarily to occlude blood vessels to stop bleeding. Fibrin also binds and reduces the activity of thrombin. This activity, sometimes referred to as antithrombin I, limits clotting. Fibrin also mediates blood platelet and endothelial cell spreading, tissue fibroblast proliferation, capillary tube formation, and angiogenesis and thereby promotes revascularization and wound healing.

Thrombin is a serine protease, an enzyme that, in humans, is encoded by the F2 gene. Prothrombin is proteolytically cleaved to form thrombin in the clotting process. Thrombin in turn acts as a serine protease that converts soluble fibrinogen into insoluble strands of fibrin, as well as catalyzing many other coagulation-related reactions.
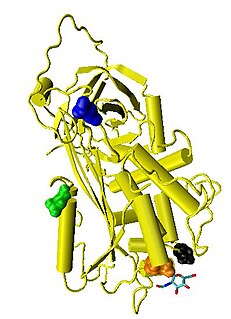
Antithrombin (AT) is a small glycoprotein that inactivates several enzymes of the coagulation system. It is a 432-amino-acid protein produced by the liver. It contains three disulfide bonds and a total of four possible glycosylation sites. α-Antithrombin is the dominant form of antithrombin found in blood plasma and has an oligosaccharide occupying each of its four glycosylation sites. A single glycosylation site remains consistently un-occupied in the minor form of antithrombin, β-antithrombin. Its activity is increased manyfold by the anticoagulant drug heparin, which enhances the binding of antithrombin to factor IIa (Thrombin) and factor Xa.
Low-molecular-weight heparin (LMWH) is a class of anticoagulant medications. They are used in the prevention of blood clots and treatment of venous thromboembolism and in the treatment of myocardial infarction.
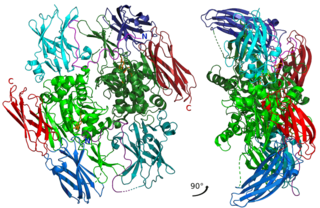
Factor XIII or fibrin stabilizing factor is a zymogen found in blood of humans and some other animals. It is activated by thrombin to factor XIIIa. Factor XIIIa is an enzyme of the blood coagulation system that crosslinks fibrin. Deficiency of XIII worsens clot stability and increases bleeding tendency.
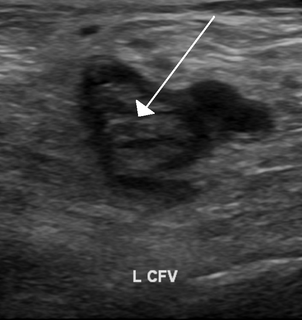
Thrombophilia is an abnormality of blood coagulation that increases the risk of thrombosis. Such abnormalities can be identified in 50% of people who have an episode of thrombosis that was not provoked by other causes. A significant proportion of the population has a detectable thrombophilic abnormality, but most of these develop thrombosis only in the presence of an additional risk factor.
Danaparoid sodium (Orgaran) is an anticoagulant with an antithrombotic action due to inhibition of thrombin generation (TGI) by two mechanisms: indirect inactivation of Factor Xa via AT and direct inhibition of thrombin activation of Factor IX. It also possesses a minor anti-thrombin activity, mediated equally via AT and Heparin Co-factor II producing a ratio of anti-Xa:IIa activity >22. [Meuleman DG. Haemostasis 1992;22:58-65 and Ofosu FA Haemostasis 1992;22:66-72]

The thrombin time (TT), also known as the thrombin clotting time (TCT), is a blood test that measures the time it takes for a clot to form in the plasma of a blood sample containing anticoagulant, after an excess of thrombin has been added. It is used to diagnose blood coagulation disorders and to assess the effectiveness of fibrinolytic therapy. This test is repeated with pooled plasma from normal patients. The difference in time between the test and the 'normal' indicates an abnormality in the conversion of fibrinogen to fibrin, an insoluble protein.
Purpura fulminans is an acute, often fatal, thrombotic disorder which manifests as blood spots, bruising and discolouration of the skin resulting from coagulation in small blood vessels within the skin and rapidly leads to skin necrosis and disseminated intravascular coagulation.

Batroxobin, also known as reptilase, is a snake venom enzyme with Venombin A activity produced by Bothrops atrox and Bothrops moojeni, venomous species of pit viper found east of the Andes in South America. It is a hemotoxin which acts as a serine protease similarly to thrombin, and has been the subject of many medical studies as a replacement of thrombin. Different enzymes, isolated from different species of Bothrops, have been called batroxobin, but unless stated otherwise, this article covers the batroxobin produced by B. moojeni, as this is the most studied variety.
The dysfibrinogenemias consist of three types of fibrinogen disorders in which a critical blood clotting factor, fibrinogen, circulates at normal levels but is dysfunctional. Congenital dysfibrinogenemia is an inherited disorder in which one of the parental genes produces an abnormal fibrinogen. This fibrinogen interferes with normal blood clotting and/or lysis of blood clots. The condition therefore may cause pathological bleeding and/or thrombosis. Acquired dysfibrinogenemia is a non-hereditary disorder in which fibrinogen is dysfunctional due to the presence of liver disease, autoimmune disease, a plasma cell dyscrasias, or certain cancers. It is associated primarily with pathological bleeding. Hereditary fibrinogen Aα-Chain amyloidosis is a sub-category of congenital dysfibrinogenemia in which the dysfunctional fibrinogen does not cause bleeding or thrombosis but rather gradually accumulates in, and disrupts the function of, the kidney.
Thromboelastometry (TEM), previously named rotational thromboelastography (ROTEG) or rotational thromboelastometry (ROTEM), is an established viscoelastic method for hemostasis testing in whole blood. It is a modification of traditional thromboelastography (TEG). TEM investigates the interaction of coagulation factors, their inhibitors, anticoagulant drugs, blood cells, specifically platelets, during clotting and subsequent fibrinolysis. The rheological conditions mimic the sluggish flow of blood in veins. While traditional thromboelastography is a global assay for blood clotting disorders and drug effects, TEM is primarily used in combination with appropriate differential assays. They allow testing in the presence of therapeutic heparin concentrations and provide differential diagnostic information to support decisions in therapy. In numerous publications the validity of the method is shown. Application of TEM at the point of care (POC) or in emergency laboratories is getting more and more popular. TEM detects both hypo- and hyperfunctional stages of the clotting process and is probably the only reliable rapid test for the diagnosis of hyperfibrinolysis. In contrast to standard clotting tests, the fibrin stabilizing effect of factor XIII contributes to the result. The rapid availability of results helps to discriminate surgical bleeding from a true haemostasis disorder and improves the therapy with blood products, factor concentrates, anticoagulants and protamine, hemostyptic and antifibrinolytic drugs. Several reports confirm that application of TEM is cost effective by reducing the consumption of blood products.
Prothrombin G20210A is a genetic condition that increases the risk of blood clots including from deep vein thrombosis, and of pulmonary embolism. One copy of the mutation increases the risk of a blood clot from 1 in 1,000 per year to 2.5 in 1,000. Two copies increases the risk to up to 20 in 1,000 per year. Most people never develop a blood clot in their lifetimes.
Direct thrombin inhibitors (DTIs) are a class of anticoagulant drugs that can be used to prevent and treat embolisms and blood clots caused by various diseases. They inhibit thrombin, a serine protease which affects the coagulation cascade in many ways. DTIs have undergone rapid development since the 90's. With technological advances in genetic engineering the production of recombinant hirudin was made possible which opened the door to this new group of drugs. Before the use of DTIs the therapy and prophylaxis for anticoagulation had stayed the same for over 50 years with the use of heparin derivatives and warfarin which have some well known disadvantages. DTIs are still under development, but the research focus has shifted towards factor Xa inhibitors, or even dual thrombin and fXa inhibitors that have a broader mechanism of action by both inhibiting factor IIa (thrombin) and Xa. A recent review of patents and literature on thrombin inhibitors has demonstrated that the development of allosteric and multi-mechanism inhibitors might lead the way to a safer anticoagulant.
Congenital hypofibrinogenemia is a rare disorder in which one of the two genes responsible for producing fibrinogen, a critical blood clotting factor, is unable to make a functional fibrinogen glycoprotein because of an inherited mutation. In consequence, liver cells, the normal site of fibrinogen production, make small amounts of this critical coagulation protein, blood levels of fibrinogen are low, and individuals with the disorder may suffer a coagulopathy, i.e. a diathesis or propensity to experience episodes of abnormal bleeding. However, individuals with congenital hypofibringenemia may also suffer episodes of abnormal blood clot formation, i.e. thrombosis. This seemingly paradoxical propensity to develop thrombosis in a disorder causing a decrease in a critical protein for blood clotting may be due to the function of fibrin to promote the lysis or disintegration of blood clots. Lower levels of fibrin may reduce the lysis of early fibrin strand depositions and thereby allow these depositions to develop into clots.










