Related Research Articles
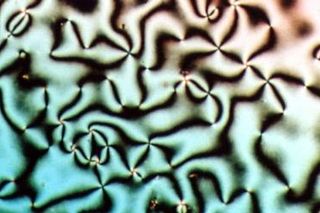
Liquid crystal (LC) is a state of matter whose properties are between those of conventional liquids and those of solid crystals. For example, a liquid crystal can flow like a liquid, but its molecules may be oriented in a common direction as in a solid. There are many types of LC phases, which can be distinguished by their optical properties. The contrasting textures arise due to molecules within one area of material ("domain") being oriented in the same direction but different areas having different orientations. An LC material may not always be in an LC state of matter.

In cell biology, a vesicle is a structure within or outside a cell, consisting of liquid or cytoplasm enclosed by a lipid bilayer. Vesicles form naturally during the processes of secretion (exocytosis), uptake (endocytosis), and the transport of materials within the plasma membrane. Alternatively, they may be prepared artificially, in which case they are called liposomes. If there is only one phospholipid bilayer, the vesicles are called unilamellar liposomes; otherwise they are called multilamellar liposomes. The membrane enclosing the vesicle is also a lamellar phase, similar to that of the plasma membrane, and intracellular vesicles can fuse with the plasma membrane to release their contents outside the cell. Vesicles can also fuse with other organelles within the cell. A vesicle released from the cell is known as an extracellular vesicle.

A micelle or micella is an aggregate of surfactant amphipathic lipid molecules dispersed in a liquid, forming a colloidal suspension. A typical micelle in water forms an aggregate with the hydrophilic "head" regions in contact with surrounding solvent, sequestering the hydrophobic single-tail regions in the micelle centre.

A liposome is a small artificial vesicle, spherical in shape, having at least one lipid bilayer. Due to their hydrophobicity and/or hydrophilicity, biocompatibility, particle size and many other properties, liposomes can be used as drug delivery vehicles for administration of pharmaceutical drugs and nutrients, such as lipid nanoparticles in mRNA vaccines, and DNA vaccines. Liposomes can be prepared by disrupting biological membranes.
Within chemical compound surfactants, pentaethylene glycol monododecyl ether (C12E5) is a nonionic surfactant. It is formed by the ethoxylation chemical reaction of dodecanol to give a material with 5 repeat units of ethylene glycol.
Poloxamers are nonionic triblock copolymers composed of a central hydrophobic chain of polyoxypropylene flanked by two hydrophilic chains of polyoxyethylene. The word poloxamer was coined by BASF inventor, Irving Schmolka, who received the patent for these materials in 1973. Poloxamers are also known by the trade names Pluronic, Kolliphor, and Synperonic.
A cell membrane defines a boundary between a cell and its environment. The primary constituent of a membrane is a phospholipid bilayer that forms in a water-based environment due to the hydrophilic nature of the lipid head and the hydrophobic nature of the two tails. In addition there are other lipids and proteins in the membrane, the latter typically in the form of isolated rafts.

Polymorphism in biophysics is the ability of lipids to aggregate in a variety of ways, giving rise to structures of different shapes, known as "phases". This can be in the form of spheres of lipid molecules (micelles), pairs of layers that face one another, a tubular arrangement (hexagonal), or various cubic phases. More complicated aggregations have also been observed, such as rhombohedral, tetragonal and orthorhombic phases.

Surfactant protein B is an essential lipid-associated protein found in pulmonary surfactant. Without it, the lung would not be able to inflate after a deep breath out. It rearranges lipid molecules in the fluid lining the lung so that tiny air sacs in the lung, called alveoli, can more easily inflate.

Lyotropic liquid crystals result when amphiphiles, which are both hydrophobic and hydrophilic, dissolve into a solution that behaves both like a liquid and a solid crystal. This liquid crystalline mesophase includes everyday mixtures like soap and water.

ATP-binding cassette sub-family A member 3 is a protein that in humans is encoded by the ABCA3 gene.

Rudolf Podgornik is a physicist. His fields of research are: physics of soft matter, physics of Coulomb fluids, physics of macromolecular interactions, Lifshitz theory of Casimir - van der Waals dispersion interaction, Casimir effect, physics of membranes, polymers and polyelectrolytes and physics of DNA, RNA and viruses.

Erich Sackmann is a German experimental physicist and a pioneer of biophysics in Europe.

The presence of ethanol can lead to the formations of non-lamellar phases also known as non-bilayer phases. Ethanol has been recognized as being an excellent solvent in an aqueous solution for inducing non-lamellar phases in phospholipids. The formation of non-lamellar phases in phospholipids is not completely understood, but it is significant that this amphiphilic molecule is capable of doing so. The formation of non-lamellar phases is significant in biomedical studies which include drug delivery, the transport of polar and non-polar ions using solvents capable of penetrating the biomembrane, increasing the elasticity of the biomembrane when it is being disrupted by unwanted substances and functioning as a channel or transporter of biomaterial.

Alexander Georgiev Petrov is a Bulgarian professor of physics and a Fellow of the Bulgarian Academy of Sciences.
In colloidal chemistry, the critical micelle concentration (CMC) of a surfactant is one of the parameters in the Gibbs free energy of micellization. The concentration at which the monomeric surfactants self-assemble into thermodynamically stable aggregates is the CMC. The Krafft temperature of a surfactant is the lowest temperature required for micellization to take place. There are many parameters that affect the CMC. The interaction between the hydrophilic heads and the hydrophobic tails play a part, as well as the concentration of salt within the solution and surfactants.

Alexandre Bouzdine (Buzdin) (in Russian - Александр Иванович Буздин; born March 16, 1954) is a French and Russian theoretical physicist in the field of superconductivity and condensed matter physics. He was awarded the Holweck Medal in physics in 2013 and obtained the Gay-Lussac Humboldt Prize in 2019 for his theoretical contributions in the field of coexistence between superconductivity and magnetism.
A unilamellar liposome is a spherical liposome, a vesicle, bounded by a single bilayer of an amphiphilic lipid or a mixture of such lipids, containing aqueous solution inside the chamber. Unilamellar liposomes are used to study biological systems and to mimic cell membranes, and are classified into three groups based on their size: small unilamellar liposomes/vesicles (SUVs) that with a size range of 20–100 nm, large unilamellar liposomes/vesicles (LUVs) with a size range of 100–1000 nm and giant unilamellar liposomes/vesicles (GUVs) with a size range of 1–200 μm. GUVs are mostly used as models for biological membranes in research work. Animal cells are 10–30 μm and plant cells are typically 10–100 μm. Even smaller cell organelles such as mitochondria are typically 1–2 μm. Therefore, a proper model should account for the size of the specimen being studied. In addition, the size of vesicles dictates their membrane curvature which is an important factor in studying fusion proteins. SUVs have a higher membrane curvature and vesicles with high membrane curvature can promote membrane fusion faster than vesicles with lower membrane curvature such as GUVs.
Roland Winter is a biophysical chemist who studies the structure, dynamics, energetics, and phase behavior of biological membranes and proteins. He was dean of the Department of Chemistry and Chemical Biology at Technical University of Dortmund, Germany.
Stéphan Fauve is a French physicist. He is a professor at the École normale supérieure (ENS) in Paris, a member of the ENS Physics Laboratory.
References
- ↑ Académie des Sciences Archived 2011-09-08 at the Wayback Machine .
- ↑ "Académie des technologies".
- ↑ Pourquoi j'ai choisi la science ? Conférence de l'UTLS [ permanent dead link ].
- ↑ Saint-Gobain Archived 2011-06-03 at the Wayback Machine .
- ↑ Conseil Scientifique International de l'ESPCI ParisTech Archived 2010-03-04 at the Wayback Machine .
- ↑ C. R. Safinya, et al., « Steric interactions in a model membrane system: a synchrotron x-ray study », Phys. Rev. Lett., 57, (1986), p. 2718
- ↑ D. Roux and C. R. Safinya, « A synchrotron x-ray study of competing undulation and electrostatic interactions in lamellar lyotropic phases », J. de Physique France, 49, (1988), p. 307
- ↑ M.E. Cates, et al., « Random surface model for the L3 phase of dilute surfactant solutions », Europhysics letters, 5, (1988), p. 733
- ↑ D. Roux, et al., « Sponge Phases in surfactant Solutions », J. Phys. Chem."Feature Article", 96, (1992), p. 4174
- ↑ O. Diat, et al., « Effect of shear on lyotropic lamellar phase », Journal de physique II France 3, (1993), p. 1427
- ↑ J. B Salmon et al., « Dynamic behaviour of the onion texture near an out of equilibrium transition », Phys; Rev., e 66, (2002), p. 031505
- ↑ P. Chenevier, et al., « Grafting molecular adresses onto biological vectors by alpha-oxohydrazone ligation : chemical caractérisation and biological efficiency », J. Am. Chem. Soc., 125, (2003), p. 52
- ↑ D. Roux et al., « Conception and realization of a non cationic non viral DNA vector », Current Medical Chemistry, 10, (2004), p. 1241-1253
- ↑ Didier Roux et Olivier Diat, « Procédé de fabrication de microcapsules ou de liposomes de taille contrôlée », Brevet Francais FRA 2689418, 1992
- ↑ D. Roux, P. Sierro, « Appareil et procédés de mesure sur un fluide en écoulement avec cisaillement », Brevet Francais délivré FR 9715577, 1997
- ↑ Médaille d'argent du CNRS [ permanent dead link ].
- ↑ "Ordre national du Mérite".