Related Research Articles

Infrared spectroscopy is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used to study and identify chemical substances or functional groups in solid, liquid, or gaseous forms. It can be used to characterize new materials or identify and verify known and unknown samples. The method or technique of infrared spectroscopy is conducted with an instrument called an infrared spectrometer which produces an infrared spectrum. An IR spectrum can be visualized in a graph of infrared light absorbance on the vertical axis vs. frequency, wavenumber or wavelength on the horizontal axis. Typical units of wavenumber used in IR spectra are reciprocal centimeters, with the symbol cm−1. Units of IR wavelength are commonly given in micrometers, symbol μm, which are related to the wavenumber in a reciprocal way. A common laboratory instrument that uses this technique is a Fourier transform infrared (FTIR) spectrometer. Two-dimensional IR is also possible as discussed below.

Spectroscopy is the field of study that measures and interprets electromagnetic spectra. In narrower contexts, spectroscopy is the precise study of color as generalized from visible light to all bands of the electromagnetic spectrum.
Fourier-transform spectroscopy is a measurement technique whereby spectra are collected based on measurements of the coherence of a radiative source, using time-domain or space-domain measurements of the radiation, electromagnetic or not. It can be applied to a variety of types of spectroscopy including optical spectroscopy, infrared spectroscopy, nuclear magnetic resonance (NMR) and magnetic resonance spectroscopic imaging (MRSI), mass spectrometry and electron spin resonance spectroscopy.

Raman spectroscopy is a spectroscopic technique typically used to determine vibrational modes of molecules, although rotational and other low-frequency modes of systems may also be observed. Raman spectroscopy is commonly used in chemistry to provide a structural fingerprint by which molecules can be identified.

Ultraviolet (UV) spectroscopy or ultraviolet–visible (UV–VIS) spectrophotometry refers to absorption spectroscopy or reflectance spectroscopy in part of the ultraviolet and the full, adjacent visible regions of the electromagnetic spectrum. Being relatively inexpensive and easily implemented, this methodology is widely used in diverse applied and fundamental applications. The only requirement is that the sample absorb in the UV-Vis region, i.e. be a chromophore. Absorption spectroscopy is complementary to fluorescence spectroscopy. Parameters of interest, besides the wavelength of measurement, are absorbance (A) or transmittance (%T) or reflectance (%R), and its change with time.

Absorption spectroscopy is spectroscopy that involves techniques that measure the absorption of electromagnetic radiation, as a function of frequency or wavelength, due to its interaction with a sample. The sample absorbs energy, i.e., photons, from the radiating field. The intensity of the absorption varies as a function of frequency, and this variation is the absorption spectrum. Absorption spectroscopy is performed across the electromagnetic spectrum.

Spectrophotometry is a branch of electromagnetic spectroscopy concerned with the quantitative measurement of the reflection or transmission properties of a material as a function of wavelength. Spectrophotometry uses photometers, known as spectrophotometers, that can measure the intensity of a light beam at different wavelengths. Although spectrophotometry is most commonly applied to ultraviolet, visible, and infrared radiation, modern spectrophotometers can interrogate wide swaths of the electromagnetic spectrum, including x-ray, ultraviolet, visible, infrared, and/or microwave wavelengths.
Absorbance is defined as "the logarithm of the ratio of incident to transmitted radiant power through a sample ". Alternatively, for samples which scatter light, absorbance may be defined as "the negative logarithm of one minus absorptance, as measured on a uniform sample". The term is used in many technical areas to quantify the results of an experimental measurement. While the term has its origin in quantifying the absorption of light, it is often entangled with quantification of light which is “lost” to a detector system through other mechanisms. What these uses of the term tend to have in common is that they refer to a logarithm of the ratio of a quantity of light incident on a sample or material to that which is detected after the light has interacted with the sample.
Diffuse reflection is the reflection of light or other waves or particles from a surface such that a ray incident on the surface is scattered at many angles rather than at just one angle as in the case of specular reflection. An ideal diffuse reflecting surface is said to exhibit Lambertian reflection, meaning that there is equal luminance when viewed from all directions lying in the half-space adjacent to the surface.
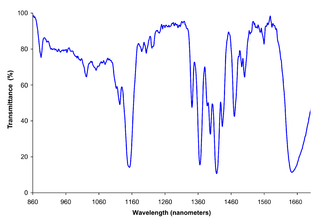
Near-infrared spectroscopy (NIRS) is a spectroscopic method that uses the near-infrared region of the electromagnetic spectrum. Typical applications include medical and physiological diagnostics and research including blood sugar, pulse oximetry, functional neuroimaging, sports medicine, elite sports training, ergonomics, rehabilitation, neonatal research, brain computer interface, urology, and neurology. There are also applications in other areas as well such as pharmaceutical, food and agrochemical quality control, atmospheric chemistry, combustion research and knowledge.

X-ray spectroscopy is a general term for several spectroscopic techniques for characterization of materials by using x-ray radiation.

Attenuated total reflection (ATR) is a sampling technique used in conjunction with infrared spectroscopy which enables samples to be examined directly in the solid or liquid state without further preparation.
Diffuse reflectance spectroscopy, or diffuse reflection spectroscopy, is a subset of absorption spectroscopy. It is sometimes called remission spectroscopy. Remission is the reflection or back-scattering of light by a material, while transmission is the passage of light through a material. The word remission implies a direction of scatter, independent of the scattering process. Remission includes both specular and diffusely back-scattered light. The word reflection often implies a particular physical process, such as specular reflection.

Dynamic light scattering (DLS) is a technique in physics that can be used to determine the size distribution profile of small particles in suspension or polymers in solution. In the scope of DLS, temporal fluctuations are usually analyzed using the intensity or photon autocorrelation function. In the time domain analysis, the autocorrelation function (ACF) usually decays starting from zero delay time, and faster dynamics due to smaller particles lead to faster decorrelation of scattered intensity trace. It has been shown that the intensity ACF is the Fourier transform of the power spectrum, and therefore the DLS measurements can be equally well performed in the spectral domain. DLS can also be used to probe the behavior of complex fluids such as concentrated polymer solutions.
Chemical imaging is the analytical capability to create a visual image of components distribution from simultaneous measurement of spectra and spatial, time information. Hyperspectral imaging measures contiguous spectral bands, as opposed to multispectral imaging which measures spaced spectral bands.

Fourier-transform infrared spectroscopy (FTIR) is a technique used to obtain an infrared spectrum of absorption or emission of a solid, liquid, or gas. An FTIR spectrometer simultaneously collects high-resolution spectral data over a wide spectral range. This confers a significant advantage over a dispersive spectrometer, which measures intensity over a narrow range of wavelengths at a time.
The Reststrahlen effect is a reflectance phenomenon in which electromagnetic radiation within a narrow energy band cannot propagate within a given medium due to a change in refractive index concurrent with the specific absorbance band of the medium in question; this narrow energy band is termed the Reststrahlen band.

Fourier transform infrared spectroscopy (FTIR) is a spectroscopic technique that has been used for analyzing the fundamental molecular structure of geological samples in recent decades. As in other infrared spectroscopy, the molecules in the sample are excited to a higher energy state due to the absorption of infrared (IR) radiation emitted from the IR source in the instrument, which results in vibrations of molecular bonds. The intrinsic physicochemical property of each particular molecule determines its corresponding IR absorbance peak, and therefore can provide characteristic fingerprints of functional groups.
The Kubelka-Munk theory, devised by Paul Kubelka and Franz Munk, is a fundamental approach to modelling the appearance of paint films. As published in 1931, the theory addresses "the question of how the color of a substrate is changed by the application of a coat of paint of specified composition and thickness, and especially the thickness of paint needed to obscure the substrate". The mathematical relationship involves just two paint-dependent constants.
The concept of the representative layer came about though the work of Donald Dahm, with the assistance of Kevin Dahm and Karl Norris, to describe spectroscopic properties of particulate samples, especially as applied to near-infrared spectroscopy. A representative layer has the same void fraction as the sample it represents and each particle type in the sample has the same volume fraction and surface area fraction as does the sample as a whole. The spectroscopic properties of a representative layer can be derived from the spectroscopic properties of particles, which may be determined by a wide variety of ways. While a representative layer could be used in any theory that relies on the mathematics of plane parallel layers, there is a set of definitions and mathematics, some old and some new, which have become part of representative layer theory.
References
- ↑ C.-P. Sherman Hsu, Ph.D. "Handbook of Instrumental Techniques for Analytical Chemistry" Prentice-Hall, New Jersey, 1997, 262.
- ↑ Fuller, Michael P.; Griffiths, Peter R. (1978). "Diffuse reflectance measurements by infrared Fourier transform spectrometry". Analytical Chemistry. 50 (13): 1906–1910. doi:10.1021/ac50035a045. ISSN 0003-2700.
- ↑ Kortüm, Gustav (1969). Reflectance spectroscopy Principles, methods, applications. Berlin: Springer. ISBN 9783642880711. OCLC 714802320.