Related Research Articles

Eicosanoids are signaling molecules made by the enzymatic or non-enzymatic oxidation of arachidonic acid or other polyunsaturated fatty acids (PUFAs) that are, similar to arachidonic acid, around 20 carbon units in length. Eicosanoids are a sub-category of oxylipins, i.e. oxidized fatty acids of diverse carbon units in length, and are distinguished from other oxylipins by their overwhelming importance as cell signaling molecules. Eicosanoids function in diverse physiological systems and pathological processes such as: mounting or inhibiting inflammation, allergy, fever and other immune responses; regulating the abortion of pregnancy and normal childbirth; contributing to the perception of pain; regulating cell growth; controlling blood pressure; and modulating the regional flow of blood to tissues. In performing these roles, eicosanoids most often act as autocrine signaling agents to impact their cells of origin or as paracrine signaling agents to impact cells in the proximity of their cells of origin. Eicosanoids may also act as endocrine agents to control the function of distant cells.

Resolvins are specialized pro-resolving mediators (SPMs) derived from omega-3 fatty acids, primarily eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), as well as from two isomers of docosapentaenoic acid (DPA), one omega-3 and one omega-6 fatty acid. As autacoids similar to hormones acting on local tissues, resolvins are under preliminary research for their involvement in promoting restoration of normal cellular function following the inflammation that occurs after tissue injury. Resolvins belong to a class of polyunsaturated fatty acid (PUFA) metabolites termed specialized proresolving mediators (SPMs).

Lipoxygenases (LOX) are a family of (non-heme) iron-containing enzymes most of which catalyze the dioxygenation of polyunsaturated fatty acids in lipids containing a cis,cis-1,4-pentadiene into cell signaling agents that serve diverse roles as autocrine signals that regulate the function of their parent cells, paracrine signals that regulate the function of nearby cells, and endocrine signals that regulate the function of distant cells.
Docosapentaenoic acid (DPA) designates any straight open chain polyunsaturated fatty acid (PUFA) which contains 22 carbons and 5 double bonds. DPA is primarily used to designate two isomers, all-cis-4,7,10,13,16-docosapentaenoic acid and all-cis-7,10,13,16,19-docosapentaenoic acid. They are also commonly termed n-6 DPA and n-3 DPA, respectively; these designations describe the position of the double bond being 6 or 3 carbons closest to the (omega) carbon at the methyl end of the molecule and is based on the biologically important difference that n-6 and n-3 PUFA are separate PUFA classes, i.e. the omega-6 fatty acids and omega-3 fatty acids, respectively. Mammals, including humans, can not interconvert these two classes and therefore must obtain dietary essential PUFA fatty acids from both classes in order to maintain normal health.
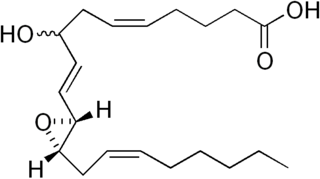
Hepoxilins (Hx) are a set of epoxyalcohol metabolites of polyunsaturated fatty acids (PUFA), i.e. they possess both an epoxide and an alcohol residue. HxA3, HxB3, and their non-enzymatically formed isomers are nonclassic eicosanoid derived from acid the (PUFA), arachidonic acid. A second group of less well studied hepoxilins, HxA4, HxB4, and their non-enzymatically formed isomers are nonclassical eicosanoids derived from the PUFA, eicosapentaenoic acid. Recently, 14,15-HxA3 and 14,15-HxB3 have been defined as arachidonic acid derivatives that are produced by a different metabolic pathway than HxA3, HxB3, HxA4, or HxB4 and differ from the aforementioned hepoxilins in the positions of their hydroxyl and epoxide residues. Finally, hepoxilin-like products of two other PUFAs, docosahexaenoic acid and linoleic acid, have been described. All of these epoxyalcohol metabolites are at least somewhat unstable and are readily enzymatically or non-enzymatically to their corresponding trihydroxy counterparts, the trioxilins (TrX). HxA3 and HxB3, in particular, are being rapidly metabolized to TrXA3, TrXB3, and TrXC3. Hepoxilins have various biological activities in animal models and/or cultured mammalian tissues and cells. The TrX metabolites of HxA3 and HxB3 have less or no activity in most of the systems studied but in some systems retain the activity of their precursor hepoxilins. Based on these studies, it has been proposed that the hepoxilins and trioxilins function in human physiology and pathology by, for example, promoting inflammation responses and dilating arteries to regulate regional blood flow and blood pressure.
Arachidonate 5-lipoxygenase, also known as ALOX5, 5-lipoxygenase, 5-LOX, or 5-LO, is a non-heme iron-containing enzyme that in humans is encoded by the ALOX5 gene. Arachidonate 5-lipoxygenase is a member of the lipoxygenase family of enzymes. It transforms essential fatty acids (EFA) substrates into leukotrienes as well as a wide range of other biologically active products. ALOX5 is a current target for pharmaceutical intervention in a number of diseases.
In biochemistry, docosanoids are signaling molecules made by the metabolism of twenty-two-carbon fatty acids (EFAs), especially the omega-3 fatty acid, Docosahexaenoic acid (DHA) by lipoxygenase, cyclooxygenase, and cytochrome P450 enzymes. Other docosanoids are metabolites of n-3 docosapentaenoic acid, n-6 DHA (i.e. 4Z,7Z,10Z,13Z,16Z-docosahexaenoic acid, and docosatetraenoic acid. Prominent docosanoid metabolites of DHA and n-3 DHA are members of the specialized proresolving mediator class of polyunsaturated fatty acid metabolites that possess potent anti-inflammation, tissue healing, and other activities.

ALOX15 is, like other lipoxygenases, a seminal enzyme in the metabolism of polyunsaturated fatty acids to a wide range of physiologically and pathologically important products. ▼ Gene Function

ALOX12, also known as arachidonate 12-lipoxygenase, 12-lipoxygenase, 12S-Lipoxygenase, 12-LOX, and 12S-LOX is a lipoxygenase-type enzyme that in humans is encoded by the ALOX12 gene which is located along with other lipoyxgenases on chromosome 17p13.3. ALOX12 is 75 kilodalton protein composed of 663 amino acids.

G protein-coupled receptor 32, also known as GPR32 or the RvD1 receptor, is a human receptor (biochemistry) belonging to the rhodopsin-like subfamily of G protein-coupled receptors.
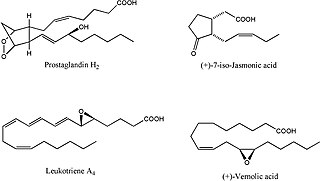
Oxylipins constitute a family of oxygenated natural products which are formed from fatty acids by pathways involving at least one step of dioxygen-dependent oxidation. Oxylipins are derived from polyunsaturated fatty acids (PUFAs) by COX enzymes (cyclooxygenases), by LOX enzymes (lipoxygenases), or by cytochrome P450 epoxygenase.
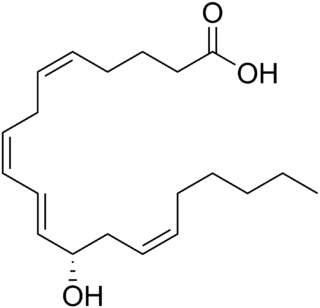
12-Hydroxyeicosatetraenoic acid (12-HETE) is a derivative of the 20 carbon polyunsaturated fatty acid, arachidonic acid, containing a hydroxyl residue at carbon 12 and a 5Z,8Z,10E,14Z Cis–trans isomerism configuration (Z=cis, E=trans) in its four double bonds. It was first found as a product of arachidonic acid metabolism made by human and bovine platelets through their 12S-lipoxygenase (i.e. ALOX12) enzyme(s). However, the term 12-HETE is ambiguous in that it has been used to indicate not only the initially detected "S" stereoisomer, 12S-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid (12(S)-HETE or 12S-HETE), made by platelets, but also the later detected "R" stereoisomer, 12(R)-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid (also termed 12(R)-HETE or 12R-HETE) made by other tissues through their 12R-lipoxygenase enzyme, ALOX12B. The two isomers, either directly or after being further metabolized, have been suggested to be involved in a variety of human physiological and pathological reactions. Unlike hormones which are secreted by cells, travel in the circulation to alter the behavior of distant cells, and thereby act as Endocrine signalling agents, these arachidonic acid metabolites act locally as Autocrine signalling and/or Paracrine signaling agents to regulate the behavior of their cells of origin or of nearby cells, respectively. In these roles, they may amplify or dampen, expand or contract cellular and tissue responses to disturbances.

Maresin 1 (MaR1 or 7R,14S-dihydroxy-4Z,8E,10E,12Z,16Z,19Z-docosahexaenoic acid) is a macrophage-derived mediator of inflammation resolution coined from macrophage mediator in resolving inflammation. Maresin 1, and more recently defined maresins, are 12-lipoxygenase-derived metabolites of the omega-3 fatty acid, docosahexaenoic acid (DHA), that possess potent anti-inflammatory, pro-resolving, protective, and pro-healing properties similar to a variety of other members of the specialized proresolving mediators (SPM) class of polyunsaturated fatty acid (PUFA) metabolites. SPM are dihydroxy, trihydroxy, and epoxy-hydroxy metabolites of long chain PUFA made by certain dioxygenase enzymes viz., cyclooxygenases and lipoxygenases. In addition to the maresins, this class of mediators includes: the 15-lipoxygenase (i.e. ALOX15 and/or possibly ALOX15B)-derived Lipoxin A4 and B4 metabolites of the omega 6 fatty acid, arachidonic acid; the cyclooxygenase 2-derived Resolvin E series metabolites of the omega 3 fatty acid, eicosapentaenoic acid; certain 15-lipoxygenase-derived Resolvin D series metabolites of DHA; certain other 15-lipoxygenase-derived protectin D1 and related metabolites of DHA; and the more recently defined and therefore less fully studied 15-lipoxygenase-derived Resolvin Dn-3DPA metabolites of the omega-3 fatty acid n-3 docosapentaenoic acid (n-3 DPA or clupanodonic acid), the cyclooxygenase 2-derived Resolvin T metabolites of this clupanodonic acid, and the 15-lipoxygenase-derived products of the N-acetylated fatty acid amide of the DHA metabolite, docosahexaenoyl ethanolamide (see resolvins).
Protectin D1 also known as neuroprotectin D1 and abbreviated most commonly as PD1 or NPD1 is a member of the class of specialized proresolving mediators. Like other members of this class of polyunsaturated fatty acid metabolites, it possesses strong anti-inflammatory, anti-apoptotic and neuroprotective activity. PD1 is an aliphatic acyclic alkene 22 carbons in length with two hydroxyl groups at the 10 and 17 carbon positions and one carboxylic acid group at the one carbon position.
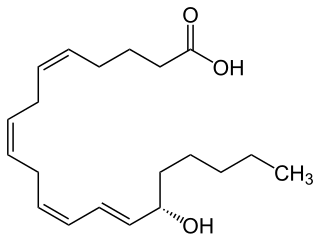
15-Hydroxyeicosatetraenoic acid (also termed 15-HETE, 15(S)-HETE, and 15S-HETE) is an eicosanoid, i.e. a metabolite of arachidonic acid. Various cell types metabolize arachidonic acid to 15(S)-hydroperoxyeicosatetraenoic acid (15(S)-HpETE). This initial hydroperoxide product is extremely short-lived in cells: if not otherwise metabolized, it is rapidly reduced to 15(S)-HETE. Both of these metabolites, depending on the cell type which forms them, can be further metabolized to 15-oxo-eicosatetraenoic acid (15-oxo-ETE), 5(S),15(S)-dihydroxy-eicosatetraenoic acid (5(S),15(S)-diHETE), 5-oxo-15(S)-hydroxyeicosatetraenoic acid (5-oxo-15(S)-HETE), a subset of specialized pro-resolving mediators viz., the lipoxins, a class of pro-inflammatory mediators, the eoxins, and other products that have less well-defined activities and functions. Thus, 15(S)-HETE and 15(S)-HpETE, in addition to having intrinsic biological activities, are key precursors to numerous biologically active derivatives.

12-Hydroxyheptadecatrienoic acid (also termed 12-HHT, 12(S)-hydroxyheptadeca-5Z,8E,10E-trienoic acid, or 12(S)-HHTrE) is a 17 carbon metabolite of the 20 carbon polyunsaturated fatty acid, arachidonic acid. It was discovered and structurally defined in 1973 by P. Wlodawer, Bengt I. Samuelsson, and M. Hamberg, as a product of arachidonic acid metabolism made by microsomes (i.e. endoplasmic reticulum) isolated from sheep seminal vesicle glands and by intact human platelets. 12-HHT is less ambiguously termed 12-(S)-hydroxy-5Z,8E,10E-heptadecatrienoic acid to indicate the S stereoisomerism of its 12-hydroxyl residue and the Z, E, and E cis-trans isomerism of its three double bonds. The metabolite was for many years thought to be merely a biologically inactive byproduct of prostaglandin synthesis. More recent studies, however, have attached potentially important activity to it.
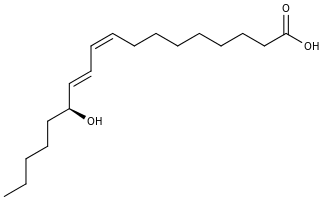
13-Hydroxyoctadecadienoic acid (13-HODE) is the commonly used term for 13(S)-hydroxy-9Z,11E-octadecadienoic acid (13(S)-HODE). The production of 13(S)-HODE is often accompanied by the production of its stereoisomer, 13(R)-hydroxy-9Z,11E-octadecadienoic acid (13(R)-HODE). The adjacent figure gives the structure for the (S) stereoisomer of 13-HODE. Two other naturally occurring 13-HODEs that may accompany the production of 13(S)-HODE are its cis-trans (i.e., 9E,11E) isomers viz., 13(S)-hydroxy-9E,11E-octadecadienoic acid (13(S)-EE-HODE) and 13(R)-hydroxy-9E,11E-octadecadienoic acid (13(R)-EE-HODE). Studies credit 13(S)-HODE with a range of clinically relevant bioactivities; recent studies have assigned activities to 13(R)-HODE that differ from those of 13(S)-HODE; and other studies have proposed that one or more of these HODEs mediate physiological and pathological responses, are markers of various human diseases, and/or contribute to the progression of certain diseases in humans. Since, however, many studies on the identification, quantification, and actions of 13(S)-HODE in cells and tissues have employed methods that did not distinguish between these isomers, 13-HODE is used here when the actual isomer studied is unclear.
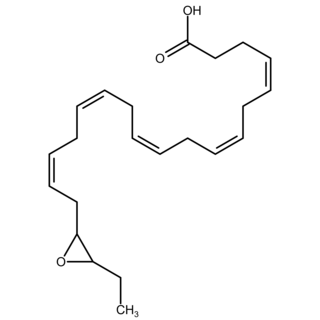
Epoxide docosapentaenoic acids are metabolites of the 22-carbon straight-chain omega-3 fatty acid, docosahexaenoic acid (DHA). Cell types that express certain cytochrome P450 (CYP) epoxygenases metabolize polyunsaturated fatty acids (PUFAs) by converting one of their double bonds to an epoxide. In the best known of these metabolic pathways, cellular CYP epoxygenases metabolize the 20-carbon straight-chain omega-6 fatty acid, arachidonic acid, to epoxyeicosatrienoic acids (EETs); another CYP epoxygenase pathway metabolizes the 20-carbon omega-3 fatty acid, eicosapentaenoic acid (EPA), to epoxyeicosatetraenoic acids (EEQs). CYP epoxygenases similarly convert various other PUFAs to epoxides. These epoxide metabolites have a variety of activities. However, essentially all of them are rapidly converted to their corresponding, but in general far less active, vicinal dihydroxy fatty acids by ubiquitous cellular soluble epoxide hydrolase. Consequently, these epoxides, including EDPs, operate as short-lived signaling agents that regulate the function of their parent or nearby cells. The particular feature of EDPs distinguishing them from EETs is that they derive from omega-3 fatty acids and are suggested to be responsible for some of the beneficial effects attributed to omega-3 fatty acids and omega-3-rich foods such as fish oil.

Epoxyeicosatetraenoic acids are a set of biologically active epoxides that various cell types make by metabolizing the omega 3 fatty acid, eicosapentaenoic acid (EPA), with certain cytochrome P450 epoxygenases. These epoxygenases can metabolize EPA to as many as 10 epoxides that differ in the site and/or stereoisomer of the epoxide formed; however, the formed EEQs, while differing in potency, often have similar bioactivities and are commonly considered together.
Specialized pro-resolving mediators are a large and growing class of cell signaling molecules formed in cells by the metabolism of polyunsaturated fatty acids (PUFA) by one or a combination of lipoxygenase, cyclooxygenase, and cytochrome P450 monooxygenase enzymes. Pre-clinical studies, primarily in animal models and human tissues, implicate SPM in orchestrating the resolution of inflammation. Prominent members include the resolvins and protectins.
References
- 1 2 3 4 Lagarde M, Véricel E, Liu M, Chen P, Guichardant M (2014). "Structure-function relationships of non-cyclic dioxygenase products from polyunsaturated fatty acids: poxytrins as a class of bioactive derivatives" (PDF). Biochimie. 107 Pt A: 91–4. doi:10.1016/j.biochi.2014.09.008. PMID 25223888. S2CID 31338779.
- 1 2 3 Balas L, Guichardant M, Durand T, Lagarde M (2014). "Confusion between protectin D1 (PD1) and its isomer protectin DX (PDX). An overview on the dihydroxy-docosatrienes described to date". Biochimie. 99: 1–7. doi:10.1016/j.biochi.2013.11.006. PMID 24262603.
- 1 2 3 Chen P, Véricel E, Lagarde M, Guichardant M (2011). "Poxytrins, a class of oxygenated products from polyunsaturated fatty acids, potently inhibit blood platelet aggregation". FASEB Journal. 25 (1): 382–8. doi: 10.1096/fj.10-161836 . PMID 20833872. S2CID 21164301.
- ↑ Sehmi R, Cromwell O, Taylor GW, Kay AB (1991). "Identification of guinea pig eosinophil chemotactic factor of anaphylaxis as leukotriene B4 and 8(S),15(S)-dihydroxy-5,9,11,13(Z,E,Z,E)-eicosatetraenoic acid". Journal of Immunology. 147 (7): 2276–83. doi: 10.4049/jimmunol.147.7.2276 . PMID 1655889. S2CID 19598067.
- 1 2 Liu M, Chen P, Véricel E, Lelli M, Béguin L, Lagarde M, Guichardant M (2013). "Characterization and biological effects of di-hydroxylated compounds deriving from the lipoxygenation of ALA". Journal of Lipid Research. 54 (8): 2083–94. doi: 10.1194/jlr.M035139 . PMC 3708359 . PMID 23740966.
- ↑ Croset M, Lagarde M (1983). "Stereospecific inhibition of PGH2-induced platelet aggregation by lipoxygenase products of icosaenoic acids". Biochemical and Biophysical Research Communications. 112 (3): 878–83. doi:10.1016/0006-291x(83)91699-6. PMID 6405751.
- ↑ Serhan CN, Gotlinger K, Hong S, Lu Y, Siegelman J, Baer T, Yang R, Colgan SP, Petasis NA (2006). "Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes". Journal of Immunology. 176 (3): 1848–59. doi: 10.4049/jimmunol.176.3.1848 . PMID 16424216.