
Venom or zootoxin is a type of toxin produced by an animal that is actively delivered through a wound by means of a bite, sting, or similar action. The toxin is delivered through a specially evolved venom apparatus, such as fangs or a stinger, in a process called envenomation. Venom is often distinguished from poison, which is a toxin that is passively delivered by being ingested, inhaled, or absorbed through the skin, and toxungen, which is actively transferred to the external surface of another animal via a physical delivery mechanism.

Tetrodotoxin (TTX) is a potent neurotoxin. Its name derives from Tetraodontiformes, an order that includes pufferfish, porcupinefish, ocean sunfish, and triggerfish; several of these species carry the toxin. Although tetrodotoxin was discovered in these fish, it is found in several other animals. It is also produced by certain infectious or symbiotic bacteria like Pseudoalteromonas, Pseudomonas, and Vibrio as well as other species found in symbiotic relationships with animals and plants.

Palytoxin, PTX or PLTX is an intense vasoconstrictor, and is considered to be one of the most poisonous non-protein substances known, second only to maitotoxin in terms of toxicity in mice.

The cabezon is a large species of sculpin native to the Pacific coast of North America. Although the genus name translates literally as "scorpion fish", true scorpionfish belong to the related family Scorpaenidae. The cabezon is the only known member of its genus.

Venomous fish are species of fish which produce strong mixtures of toxins harmful to humans which they deliberately deliver by means of a bite, sting, or stab, resulting in an envenomation. As a contrast, poisonous fish also produce a strong toxin, but they do not bite, sting, or stab to deliver the toxin, instead being poisonous to eat because the human digestive system does not destroy the toxin they contain in their bodies. Venomous fish do not necessarily cause poisoning if they are eaten, as the digestive system often destroys the venom.
Taipoxin is a potent myo- and neurotoxin that was isolated from the venom of the coastal taipan Oxyuranus scutellatus or also known as the common taipan. Taipoxin like many other pre-synaptic neurotoxins are phospholipase A2 (PLA2) toxins, which inhibit/complete block the release of the motor transmitter acetylcholine and lead to death by paralysis of the respiratory muscles (asphyxia). It is the most lethal neurotoxin isolated from any snake venom to date.

Pardachirus marmoratus, the finless sole, speckled sole or Red Sea Moses sole, is a species of flatfish native to the western Indian Ocean.

The Sierra newt is a newt found west of the Sierra Nevada, from Shasta county to Tulare County, in California, Western North America.

Diamphidia, or Bushman arrow-poison beetle, is an African genus of flea beetles, in the family Chrysomelidae.
Diamphotoxin is a toxin produced by larvae and pupae of the beetle genus Diamphidia. Diamphotoxin is a hemolytic, cardiotoxic, and highly labile single-chain polypeptide bound to a protein that protects it from deactivation.
Birtoxin is a neurotoxin from the venom of the South African Spitting scorpion. By changing sodium channel activation, the toxin promotes spontaneous and repetitive firing much like pyrethroid insecticides do
Bestoxin is a neurotoxin from the venom of the South African spitting scorpion Parabuthus transvaalicus. Most likely, it targets sodium channel function, thus promoting spontaneous and repetitive neuronal firing. Following injection into mice, it causes non-lethal writhing behaviour.
Ichthyotoxins are compounds which are either toxic to fish, or are toxins produced by fish. The former include the algae-produced euglenophycin and prymnesins, which can cause large-scale fish deaths. The latter includes ostracitoxin, produced by boxfish. Many toxin-producing algal species can be found both in marine and fresh water environments when the algae are in bloom. Ichthyotoxic poisoning in humans can cause symptoms ranging in severity dependent on how much toxin was consumed. The symptoms of an ichthyotoxin poisoning from fish venoms can include headache, vomiting, diarrhea, dizziness, and drop in blood pressure.

Diamphidia nigroornata or Bushman arrow-poison beetle, is an African leaf beetle species in the genus Diamphidia.
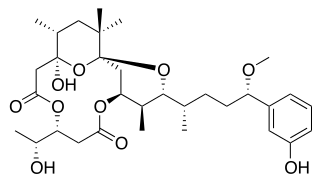
Debromoaplysiatoxin is a toxic agent produced by the blue-green alga Lyngbya majuscula. This alga lives in marine waters and causes seaweed dermatitis. Furthermore, it is a tumor promoter which has an anti-proliferative activity against various cancer cell lines in mice.
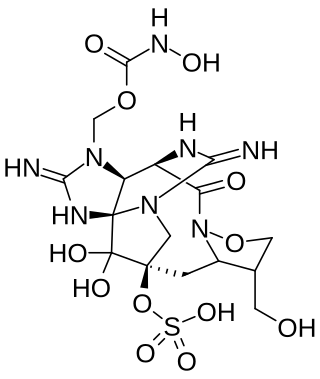
Zetekitoxin AB (ZTX) is a guanidine alkaloid found in the Panamanian golden frog Atelopus zeteki. It is an extremely potent neurotoxin.

Guanitoxin (GNT), formerly known as anatoxin-a(S) "Salivary", is a naturally occurring cyanotoxin commonly isolated from cyanobacteria. It is a potent covalent acetylcholinesterase inhibitor, and thus a potent rapid acting neurotoxin which in cases of severe exposure can lead to death. Guanitoxin was first structurally characterized in 1989, and consists of a cyclic N-hydroxyguanine organophosphate with a phosphate ester moiety.
Grammistins are peptide toxins synthesised by glands in the skin of soapfishes of the tribes Grammistini and Diploprionini which are both classified within the grouper subfamily Epinephelinae, a part of the family Serranidae. Grammistin has a hemolytic and ichthyotoxic action. The grammistins have secondary structures and biological effects comparable to other classes of peptide toxins, melittin from the bee stings and pardaxins which are secreted in the skin of two sole species. A similar toxin has been found to be secreted in the skin of some clingfishes.
Ts8 is a neurotoxin present in the venom of the Brazilian yellow scorpion, Tityus serrulatus. Ts8 is a selective inhibitor of the voltage-gated potassium channel Kv4.2

Scaritoxin, a potent toxic substance, is a ciguatoxin with molecular formula C60H84O16. Scaritoxin is also referred to as ciguaotoxin 4A, CTX4A. Like other ciguatoxins, CTX4A is produced by dinoflagellate Gambierdiscus toxicus and isolated from poisonous fish.












