Lignin is a class of complex organic polymers that form key structural materials in the support tissues of most plants. Lignins are particularly important in the formation of cell walls, especially in wood and bark, because they lend rigidity and do not rot easily. Chemically, lignins are polymers made by cross-linking phenolic precursors.
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during a non-stereospecific creation of a new stereocenter or during a non-stereospecific transformation of a pre-existing one. The selectivity arises from differences in steric and electronic effects in the mechanistic pathways leading to the different products. Stereoselectivity can vary in degree but it can never be total since the activation energy difference between the two pathways is finite: both products are at least possible and merely differ in amount. However, in favorable cases, the minor stereoisomer may not be detectable by the analytic methods used.
The lignans are a large group of low molecular weight polyphenols found in plants, particularly seeds, whole grains, and vegetables. The name derives from the Latin word for "wood". Lignans are precursors to phytoestrogens. They may play a role as antifeedants in the defense of seeds and plants against herbivores.

Podophyllotoxin (PPT) is the active ingredient in Podofilox, which is a medical cream that is used to treat genital warts and molluscum contagiosum. It is not recommended in HPV infections without external warts. It can be applied either by a healthcare provider or the person themselves.
Guaiacol is an organic compound with the formula C6H4(OH)(OCH3). It is a phenolic compound containing a methoxy functional group. Guaiacol appears as a viscous colorless oil, although aged or impure samples are often yellowish. It occurs widely in nature and is a common product of the pyrolysis of wood.

Coniferyl alcohol is an organic compound with the formula HO(CH3O)C6H3CH=CHCH2OH. A colourless or white solid, it is one of the monolignols, produced via the phenylpropanoid biochemical pathway. When copolymerized with related aromatic compounds, coniferyl alcohol forms lignin or lignans. Coniferin is a glucoside of coniferyl alcohol. Coniferyl alcohol is an intermediate in biosynthesis of eugenol and of stilbenoids and coumarin. Gum benzoin contains significant amount of coniferyl alcohol and its esters. It is found in both gymnosperm and angiosperm plants. Sinapyl alcohol and paracoumaryl alcohol, the other two lignin monomers, are found in angiosperm plants and grasses.

The phenylpropanoids are a diverse family of organic compounds that are biosynthesized by plants from the amino acids phenylalanine and tyrosine in the shikimic acid pathway. Their name is derived from the six-carbon, aromatic phenyl group and the three-carbon propene tail of coumaric acid, which is the central intermediate in phenylpropanoid biosynthesis. From 4-coumaroyl-CoA emanates the biosynthesis of myriad natural products including lignols, flavonoids, isoflavonoids, coumarins, aurones, stilbenes, catechin, and phenylpropanoids. The coumaroyl component is produced from cinnamic acid.

Syringol is the organic compound with the formula HO(CH3O)2C6H3. The molecule is a phenol, with methoxy groups in the flanking (2 and 6) positions. It is the symmetrically dimethylated derivative of pyrogallol. It is a colorless solid, although typical samples are brown owing to air-oxidized impurities. Together with guaiacol, syringol and its derivatives are produced by the pyrolysis of lignin. Specifically, syringol is derived from the thermal decomposition of the sinapyl alcohol component. As such, syringol is an important component of wood smoke.
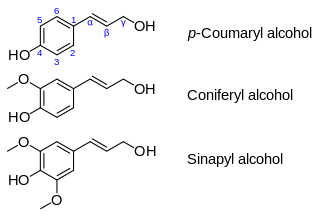
Monolignols, also called lignols, are the source materials for biosynthesis of both lignans and lignin and consist mainly of paracoumaryl alcohol (H), coniferyl alcohol (G) and sinapyl alcohol (S). These monolignols differ in their degree of methoxylation of the aromatic ring.

Sinapyl alcohol is an organic compound structurally related to cinnamic acid. It is biosynthetized via the phenylpropanoid biochemical pathway, its immediate precursor being sinapaldehyde. This phytochemical is one of the monolignols, which are precursor to lignin or lignans. It is also a biosynthetic precursor to various stilbenoids and coumarins.
In enzymology, a dihydrokaempferol 4-reductase (EC 1.1.1.219) is an enzyme that catalyzes the chemical reaction

Cinnamoyl-CoA reductase (EC 1.2.1.44), systematically named cinnamaldehyde:NADP+ oxidoreductase (CoA-cinnamoylating) but commonly referred to by the acronym CCR, is an enzyme that catalyzes the reduction of a substituted cinnamoyl-CoA to its corresponding cinnamaldehyde, utilizing NADPH and H+ and releasing free CoA and NADP+ in the process. Common biologically relevant cinnamoyl-CoA substrates for CCR include p-coumaroyl-CoA and feruloyl-CoA, which are converted into p-coumaraldehyde and coniferaldehyde, respectively, though most CCRs show activity toward a variety of other substituted cinnamoyl-CoA's as well. Catalyzing the first committed step in monolignol biosynthesis, this enzyme plays a critical role in lignin formation, a process important in plants both for structural development and defense response.

Forsythia suspensa, commonly known as weeping forsythia or golden-bell, is a species of flowering plant in the family Oleaceae. It is native to China.

Forsythia × intermedia, or border forsythia, is an ornamental deciduous shrub of garden origin.

Sinapaldehyde is an organic compound with the formula HO(CH3O)2C6H2CH=CHCHO. It is a derivative of cinnamaldehyde, featuring one hydroxy group and two methoxy groups as substituents. It is an intermediate in the formation of sinapyl alcohol, a lignol that is a major precursor to lignin.

Coniferyl aldehyde is an organic compound with the formula HO(CH3O)C6H3CH=CHCHO. It is a derivative of cinnamaldehyde, featuring 4-hydroxy and 3-methoxy substituents. It is a major precursor to lignin.

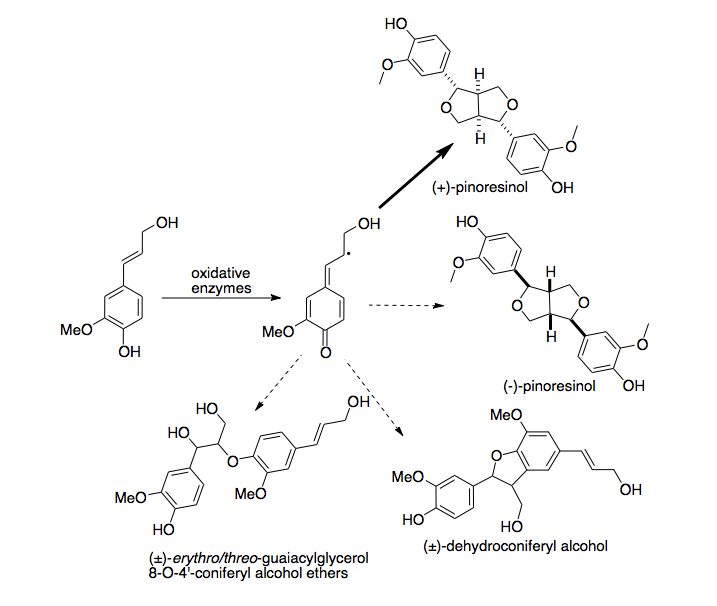
Pinoresinol is a tetrahydrofuran lignan found in Styrax sp., Forsythia suspensa, and in Forsythia koreana. It is also found in the caterpillar of the cabbage butterfly, Pieris rapae where it serves as a defence against ants.
Eugenol synthase (EC 1.1.1.318, LtCES1, EGS1, EGS2) is an enzyme with systematic name eugenol:NADP+ oxidoreductase (coniferyl ester reducing). This enzyme catalyses the following chemical reaction: eugenol + a carboxylate + NADP+ a coniferyl ester + NADPH + H+
Secoisolariciresinol dehydrogenase (EC 1.1.1.331) is an enzyme with the systematic name (-)-secoisolariciresinol:NAD+ oxidoreductase. This enzyme catalyses the following chemical reaction:

Silychristin is a natural product and one of the constituents of silymarin, the standardized, active extract of the fruit of milk thistle, Silybum marianum. It is the second most abundant constituent in silymarin, after silybin. Silychristin is a flavonolignan, along with many other silymarin constituents, meaning it is composed up of a flavonoid and a lignan. It is estimated that up to 65–80% of silymarin extract is made up of flavonolignans, like silychristin, which give silymarin its well known potent antioxidant and hepatoprotective properties. Silychristin can exist as two stereoisomers, silychristin A and silychristin B. The marianum variety of S. marianum includes silychristin A as a major flavonolignan constituent, while the lesser known and studied albiflorum variety includes unique flavonolignans, including silyhermin, (–)-silandrin, and (+)-silymonin.