
A neurotransmitter is a signaling molecule secreted by a neuron to affect another cell across a synapse. The cell receiving the signal, or target cell, may be another neuron, but could also be a gland or muscle cell.
An inhibitory postsynaptic potential (IPSP) is a kind of synaptic potential that makes a postsynaptic neuron less likely to generate an action potential. The opposite of an inhibitory postsynaptic potential is an excitatory postsynaptic potential (EPSP), which is a synaptic potential that makes a postsynaptic neuron more likely to generate an action potential. IPSPs can take place at all chemical synapses, which use the secretion of neurotransmitters to create cell-to-cell signalling. EPSPs and IPSPs compete with each other at numerous synapses of a neuron. This determines whether an action potential occurring at the presynaptic terminal produces an action potential at the postsynaptic membrane. Some common neurotransmitters involved in IPSPs are GABA and glycine.

The periaqueductal gray (PAG), also known as the central gray, is a brain region that plays a critical role in autonomic function, motivated behavior and behavioural responses to threatening stimuli. PAG is also the primary control center for descending pain modulation. It has enkephalin-producing cells that suppress pain.

The reticular formation is a set of interconnected nuclei that is located in the brainstem, hypothalamus, and other regions. It is not anatomically well defined, because it includes neurons located in different parts of the brain. The neurons of the reticular formation make up a complex set of networks in the core of the brainstem that extend from the upper part of the midbrain to the lower part of the medulla oblongata.
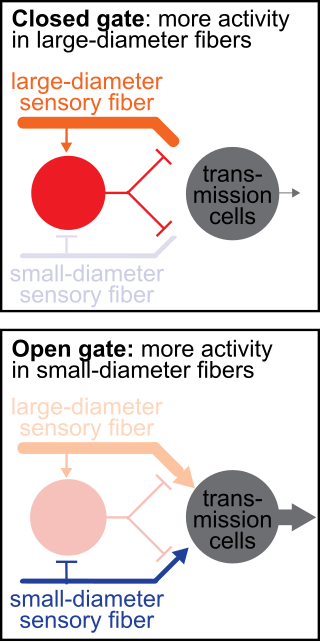
The gate control theory of pain asserts that non-painful input closes the nerve "gates" to painful input, which prevents pain sensation from traveling to the central nervous system.
Neuromodulation is the physiological process by which a given neuron uses one or more chemicals to regulate diverse populations of neurons. Neuromodulators typically bind to metabotropic, G-protein coupled receptors (GPCRs) to initiate a second messenger signaling cascade that induces a broad, long-lasting signal. This modulation can last for hundreds of milliseconds to several minutes. Some of the effects of neuromodulators include altering intrinsic firing activity, increasing or decreasing voltage-dependent currents, altering synaptic efficacy, increasing bursting activity and reconfiguring synaptic connectivity.

The nucleus raphe magnus (NRM) is one of the seven raphe nuclei. It is situated in the pons in the brainstem, just rostral to the nucleus raphe obscurus.

The apex of the posterior grey column, one of the three grey columns of the spinal cord, is capped by a V-shaped or crescentic mass of translucent, gelatinous neuroglia, termed the substantia gelatinosa of Rolando, which contains both neuroglia cells, and small neurons. The gelatinous appearance is due to an abundance of neuropil with a very low concentration of myelinated fibers. It extends the entire length of the spinal cord and into the medulla oblongata where it becomes the spinal trigeminal nucleus.

Medium spiny neurons (MSNs), also known as spiny projection neurons (SPNs), are a special type of inhibitory GABAergic neuron representing approximately 90% of neurons within the human striatum, a basal ganglia structure. Medium spiny neurons have two primary phenotypes : D1-type MSNs of the direct pathway and D2-type MSNs of the indirect pathway. Most striatal MSNs contain only D1-type or D2-type dopamine receptors, but a subpopulation of MSNs exhibit both phenotypes.

Endomorphins are considered to be natural opioid neuropeptides central to pain relief. The two known endomorphins, endomorphin-1 and endomorphin-2, are tetrapeptides, consisting of Tyr-Pro-Trp-Phe and Tyr-Pro-Phe-Phe amino acid sequences respectively. These sequences fold into tertiary structures with high specificity and affinity for the μ-opioid receptor, binding it exclusively and strongly. Bound μ-opioid receptors typically induce inhibitory effects on neuronal activity. Endomorphin-like immunoreactivity exists within the central and peripheral nervous systems, where endomorphin-1 appears to be concentrated in the brain and upper brainstem, and endomorphin-2 in the spinal cord and lower brainstem. Because endomorphins activate the μ-opioid receptor, which is the target receptor of morphine and its derivatives, endomorphins possess significant potential as analgesics with reduced side effects and risk of addiction.
The lateral hypothalamus (LH), also called the lateral hypothalamic area (LHA), contains the primary orexinergic nucleus within the hypothalamus that widely projects throughout the nervous system; this system of neurons mediates an array of cognitive and physical processes, such as promoting feeding behavior and arousal, reducing pain perception, and regulating body temperature, digestive functions, and blood pressure, among many others. Clinically significant disorders that involve dysfunctions of the orexinergic projection system include narcolepsy, motility disorders or functional gastrointestinal disorders involving visceral hypersensitivity, and eating disorders.
The gigantocellular reticular nucleus is the (efferent/motor) medial zone of the reticular formation of the caudal pons and rostral medulla oblongata. It consists of a substantial number of giant neurons, but also contains small and medium sized neurons.
The spinoreticular tract is a partially decussating (crossed-over) four-neuron sensory pathway of the central nervous system. The tract transmits slow nociceptive/pain information from the spinal cord to reticular formation which in turn relays the information to the thalamus via reticulothalamic fibers as well as to other parts of the brain. Most (85%) second-order axons arising from sensory C first-order fibers ascend in the spinoreticular tract - it is consequently responsible for transmiting "slow", dull, poorly-localised pain. By projecting to the reticular activating system (RAS), the tract also mediates arousal/alertness in response to noxious (harmful) stimuli. The tract is phylogenetically older than the spinothalamic ("neospinothalamic") tract.
The Golgi tendon reflex (also called inverse stretch reflex, autogenic inhibition, tendon reflex) is an inhibitory effect on the muscle resulting from the muscle tension stimulating Golgi tendon organs (GTO) of the muscle, and hence it is self-induced. The reflex arc is a negative feedback mechanism preventing too much tension on the muscle and tendon. When the tension is extreme, the inhibition can be so great it overcomes the excitatory effects on the muscle's alpha motoneurons causing the muscle to suddenly relax. This reflex is also called the inverse myotatic reflex, because it is the inverse of the stretch reflex.
Sleep onset is the transition from wakefulness into sleep. Sleep onset usually transits into non-rapid eye movement sleep but under certain circumstances it is possible to transit from wakefulness directly into rapid eye movement sleep.

Clinical neurochemistry is the field of neurological biochemistry which relates biochemical phenomena to clinical symptomatic manifestations in humans. While neurochemistry is mostly associated with the effects of neurotransmitters and similarly functioning chemicals on neurons themselves, clinical neurochemistry relates these phenomena to system-wide symptoms. Clinical neurochemistry is related to neurogenesis, neuromodulation, neuroplasticity, neuroendocrinology, and neuroimmunology in the context of associating neurological findings at both lower and higher level organismal functions.

Central pattern generators are biological neural networks organized to produce any rhythmic output without requiring a rhythmic input. In mammals, locomotor CPGs are organized in the lumbar and cervical segments of the spinal cord, and are used to control rhythmic muscle output in the arms and legs. Certain areas of the brain initiate the descending neural pathways that ultimately control and modulate the CPG signals. In addition to this direct control, there exist different feedback loops that coordinate the limbs for efficient locomotion and allow for the switching of gaits under appropriate circumstances.

A spinal interneuron, found in the spinal cord, relays signals between (afferent) sensory neurons, and (efferent) motor neurons. Different classes of spinal interneurons are involved in the process of sensory-motor integration. Most interneurons are found in the grey column, a region of grey matter in the spinal cord.
The raphespinal tract is an unmyelinated descending serotonergic tract involved in pain modulation. It is a descending pain-inhibiting pathway; it is a component of the reticulospinal tract.
The parafacial zone (PZ) is a brain structure located in the brainstem within the medulla oblongata believed to be heavily responsible for non-rapid eye movement (non-REM) sleep regulation, specifically for inducing slow-wave sleep.












