Related Research Articles

Sir Peter Karel, Baron Piot, is a Belgian-British microbiologist known for his research into Ebola and AIDS.

Ebola, also known as Ebola virus disease (EVD) and Ebola hemorrhagic fever (EHF), is a viral hemorrhagic fever in humans and other primates, caused by ebolaviruses. Symptoms typically start anywhere between two days and three weeks after infection. The first symptoms are usually fever, sore throat, muscle pain, and headaches. These are usually followed by vomiting, diarrhoea, rash and decreased liver and kidney function, at which point some people begin to bleed both internally and externally. It kills between 25% and 90% of those infected – about 50% on average. Death is often due to shock from fluid loss, and typically occurs between six and 16 days after the first symptoms appear. Early treatment of symptoms increases the survival rate considerably compared to late start. An Ebola vaccine was approved by the US FDA in December 2019.

Brincidofovir, sold under the brand name Tembexa, is an antiviral drug used to treat smallpox. Brincidofovir is a prodrug of cidofovir. Conjugated to a lipid, the compound is designed to release cidofovir intracellularly, allowing for higher intracellular and lower plasma concentrations of cidofovir, effectively increasing its activity against dsDNA viruses, as well as oral bioavailability.

The 2013–2016 epidemic of Ebola virus disease, centered in Western Africa, was the most widespread outbreak of the disease in history. It caused major loss of life and socioeconomic disruption in the region, mainly in Guinea, Liberia and Sierra Leone. The first cases were recorded in Guinea in December 2013; later, the disease spread to neighbouring Liberia and Sierra Leone, with minor outbreaks occurring in Nigeria and Mali. Secondary infections of medical workers occurred in the United States and Spain. In addition, isolated cases were recorded in Senegal, the United Kingdom and Italy. The number of cases peaked in October 2014 and then began to decline gradually, following the commitment of substantial international resources.

ZMapp is an experimental biopharmaceutical drug comprising three chimeric monoclonal antibodies under development as a treatment for Ebola virus disease. Two of the three components were originally developed at the Public Health Agency of Canada's National Microbiology Laboratory (NML), and the third at the U.S. Army Medical Research Institute of Infectious Diseases; the cocktail was optimized by Gary Kobinger, a research scientist at the NML and underwent further development under license by Mapp Biopharmaceutical. ZMapp was first used on humans during the Western African Ebola virus epidemic, having only been previously tested on animals and not yet subjected to a randomized controlled trial. The National Institutes of Health (NIH) ran a clinical trial starting in January 2015 with subjects from Sierra Leone, Guinea, and Liberia aiming to enroll 200 people, but the epidemic waned and the trial closed early, leaving it too statistically underpowered to give a meaningful result about whether ZMapp worked.

Recombinant vesicular stomatitis virus–Zaire Ebola virus (rVSV-ZEBOV), also known as Ebola Zaire vaccine live and sold under the brand name Ervebo, is an Ebola vaccine for adults that prevents Ebola caused by the Zaire ebolavirus. When used in ring vaccination, rVSV-ZEBOV has shown a high level of protection. Around half the people given the vaccine have mild to moderate adverse effects that include headache, fatigue, and muscle pain.

An epidemic of Ebola virus disease in Guinea from 2013 to 2016 represents the first ever outbreak of Ebola in a West African country. Previous outbreaks have been confined to several countries in Sub-Saharan Africa.

An epidemic of Ebola virus disease occurred in Liberia from 2014 to 2015, along with the neighbouring countries of Guinea and Sierra Leone. The first cases of virus were reported by late March 2014. The Ebola virus, a biosafety level four pathogen, is an RNA virus discovered in 1976.

Ebola vaccines are vaccines either approved or in development to prevent Ebola. As of 2022, there are only vaccines against the Zaire ebolavirus. The first vaccine to be approved in the United States was rVSV-ZEBOV in December 2019. It had been used extensively in the Kivu Ebola epidemic under a compassionate use protocol. During the early 21st century, several vaccine candidates displayed efficacy to protect nonhuman primates against lethal infection.

Organizations from around the world responded to the West African Ebola virus epidemic. In July 2014, the World Health Organization (WHO) convened an emergency meeting with health ministers from eleven countries and announced collaboration on a strategy to co-ordinate technical support to combat the epidemic. In August, they declared the outbreak an international public health emergency and published a roadmap to guide and coordinate the international response to the outbreak, aiming to stop ongoing Ebola transmission worldwide within 6–9 months. In September, the United Nations Security Council declared the Ebola virus outbreak in the West Africa subregion a "threat to international peace and security" and unanimously adopted a resolution urging UN member states to provide more resources to fight the outbreak; the WHO stated that the cost for combating the epidemic will be a minimum of $1 billion.

There is no cure or specific treatment for the Ebola virus disease that is currently approved for market, although various experimental treatments are being developed. For past and current Ebola epidemics, treatment has been primarily supportive in nature.
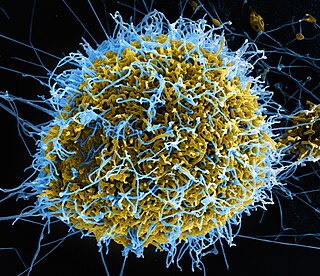
Post-Ebola virus syndrome is a post-viral syndrome affecting those who have recovered from infection with Ebola. Symptoms include joint and muscle pain, eye problems, including blindness, various neurological problems, and other ailments, sometimes so severe that the person is unable to work. Although similar symptoms had been reported following previous outbreaks in the last 20 years, health professionals began using the term in 2014 when referring to a constellation of symptoms seen in people who had recovered from an acute attack of Ebola disease.
Ansuvimab, sold under the brand name Ebanga, is a monoclonal antibody medication for the treatment of Zaire ebolavirus (Ebolavirus) infection.
Science diplomacy is the collaborative efforts by local and global entities to solve global issues using science and technology as a base. In science diplomacy, collaboration takes place to advance science but science can also be used to facilitate diplomatic relations. This allows even conflicting nations to come together through science to find solutions to global issues. Global organizations, researchers, public health officials, countries, government officials, and clinicians have previously worked together to create effective measures of infection control and subsequent treatment. They continue to do so through sharing of resources, research data, ideas, and by putting into effect laws and regulations that can further advance scientific research. Without the collaborative efforts of such entities, the world would not have the vaccines and treatments we now possess for diseases that were once considered deadly such as tuberculosis, tetanus, polio, influenza, etc. Historically, science diplomacy has proved successful in diseases such as SARS, Ebola, Zika and continues to be relevant during the COVID-19 pandemic today.

On 7 February 2021, the Congolese health ministry announced that a new case of Ebola near Butembo, North Kivu had been detected the previous day. The case was a 42-year-old woman who had symptoms of Ebola in Biena on 1 February 2021. A few days after, she died in a hospital in Butembo. The WHO said that more than 70 people who had contact with the woman had been tracked.

John R. Mascola is an American physician-scientist, immunologist and infectious disease specialist. He was the director of the Vaccine Research Center (VRC), part of the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH). He also served as a principal advisor to Anthony Fauci, director of NIAID, on vaccines and biomedical research affairs. Mascola is the current Chief Scientific Officer for ModeX Therapeutics.
Gary P. Kobinger is a Canadian immunologist and virologist who is currently the director at the Galveston National Laboratory at the University of Texas. He has held previous professorships at Université Laval, the University of Manitoba, and the University of Pennsylvania. Additionally, he was the chief of the Special Pathogens Unit at the National Microbiology Laboratory (NML) of the Public Health Agency of Canada (PHAC) in Winnipeg, Manitoba, for eight years. Kobinger is known for his critical role in the development of both an effective Ebola vaccine and treatment. His work focuses on the development and evaluation of new vaccine platforms and immunological treatments against emerging and re-emerging viruses that are dangerous to human health.
The Wyss Institute for Biologically Inspired Engineering is a cross-disciplinary research institute at Harvard University focused on bridging the gap between academia and industry by drawing inspiration from nature's design principles to solve challenges in health care and the environment. It is focused on the field of biologically inspired engineering to be distinct from bioengineering and biomedical engineering. The institute also has a focus on applications, intellectual property generation, and commercialization. The Wyss Institute is located in Boston's Longwood Medical Area and has 375 full-time staff. The Wyss is organized around eight focus areas, each of which integrate faculty, postdocs, fellows, and staff scientists. The focus areas are bioinspired therapeutics & diagnostics, diagnostics accelerator, immuno-materials, living cellular devices, molecular robotics, 3D organ engineering, predictive bioanalytics and synthetic biology.
Events in the year 2016 in Liberia.
References
- 1 2 3 "Dougbeh-Chris Nyan, M.D." (PDF). U.S. House of Representatives Document Repository. Retrieved 2017-10-18.
- 1 2 3 "Liberian Scientist, Inventor Dr. Dougbeh Nyan Is 'July 26' National Orator". Liberia News Agency (Monrovia). 2016-07-22. Retrieved 2017-10-18.
- ↑ "Dougbeh-Chris Nyan | Innovation Prize for Africa". innovationprizeforafrica.org. Retrieved 2017-10-18.
- 1 2 3 "FDA Returning Patent to Liberian Scientist Following US Congressional Investigation -". 2016-10-15. Retrieved 2017-10-18.
- 1 2 Columnist, Joe Davidson | (2016-03-28). "Did FDA remove scientist's name from article and invention in retaliation?". Washington Post. ISSN 0190-8286 . Retrieved 2017-10-18.
- ↑ "10 African innovators with game-changing ideas". How We Made It In Africa. 2017-06-19. Retrieved 2017-10-18.
- ↑ "Dr. Dougbeh Chris Nyan Wins 2017 African Innovation Special Prize Award for Social Impact -". 2017-07-21. Retrieved 2017-10-18.