Douglas "Doug" H. Turner is an American chemist and Professor of Chemistry at the University of Rochester.
Contents
Douglas H. Turner | |
|---|---|
| Website | http://rna.chem.rochester.edu/ |
Douglas "Doug" H. Turner is an American chemist and Professor of Chemistry at the University of Rochester.
Douglas H. Turner | |
|---|---|
| Website | http://rna.chem.rochester.edu/ |
Turner grew up in Brooklyn, New York.
Turner attended Harvard College, where he graduated cum laude in Chemistry and was commissioned as a Second Lieutenant in the U.S. Army. He did his graduate work in the Chemistry Departments of Columbia University and Brookhaven National Labs, where he worked with George Flynn and Norman Sutin to develop the Raman laser temperature jump method for measuring kinetics on a nanosecond time scale. During this period, he also spent three months in Anniston, Alabama taking the Officer's Basic Course of the Army's Chemical Corp. Deciding that he liked science more than war, he turned down the opportunity to continue as an active duty officer and went to the University of California at Berkeley to postdoc with Ignacio Tinoco, Jr. There, he invented fluorescence detected circular dichroism for measuring the optical activity of the fluorescent component of a solution.
In 1975, Turner joined the faculty of the Chemistry Department at the University of Rochester, where he is still a Professor. Turner was also lucky to be part of the academic family of Tom Cech (Nobel Prize in Chemistry, 1989) during 2 sabbatical years at the University of Colorado at Boulder. Turner has been unusually lucky with his own academic family of 8 postdocs, 49 students who have graduated with Ph.D.'s, and his other collaborators. Together, they have discovered many of the fundamental principles that determine RNA structure. [1] These principles, occasionally dubbed "Turner Rules", [2] are used in many RNA structure prediction algorithms. This has helped advance methods for predicting RNA structure from sequence, as well as RNA-RNA interactions: e.g. miRNA or siRNA target binding. Methods using the "Turner Rules" are widely used by biochemists and biologists. [3] [4] In his own lab, these methods were used to discover potentially medically important RNA structures in influenza virus [5] including an RNA pseudoknot that may play a role in regulating splicing at the Influenza A Segment 7 3' Splice Site.
Recently, Turner and collaborators have used Nuclear Magnetic Resonance and Molecular Dynamics simulations of short RNAs to test understanding of the sequence dependence of stacking interactions. [6] [7] Much remains to be discovered.
Papers coauthored by Turner have been cited over 20,000 times (Google Scholar). The work has also been recognized by Sloan and Guggenheim Fellowships, election as a Fellow of the American Association for the Advancement of Science (AAAS), selection by the American Chemical Society as a Gordon Hammes Lecturer, continuous funding of an NIH grant from 1976 to 2019, and coauthorship of more than 250 papers. With Ryszard Kierzek from the Institute of Bioorganic Chemistry in Poznan, he shared the AAAS Poland-US Science Award in 2016. In 2023 Turner won the RNA Society / Cold Spring Harbor Laboratory Press "Distinguished Research Mentor Award.” [8]
Turner has also served the scientific community by often teaching the first year undergraduate Chemistry course and the graduate Biophysical Chemistry course, by being a member of several NIH Study Sections, the Advisory Board of the Institute of Bioorganic Chemistry in Poznan, and the editorial board of the Biophysical Journal. He also co-chaired a Nucleic Acids Gordon Conference.

Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity.

Biophysics is an interdisciplinary science that applies approaches and methods traditionally used in physics to study biological phenomena. Biophysics covers all scales of biological organization, from molecular to organismic and populations. Biophysical research shares significant overlap with biochemistry, molecular biology, physical chemistry, physiology, nanotechnology, bioengineering, computational biology, biomechanics, developmental biology and systems biology.
Oligonucleotides are short DNA or RNA molecules, oligomers, that have a wide range of applications in genetic testing, research, and forensics. Commonly made in the laboratory by solid-phase chemical synthesis, these small fragments of nucleic acids can be manufactured as single-stranded molecules with any user-specified sequence, and so are vital for artificial gene synthesis, polymerase chain reaction (PCR), DNA sequencing, molecular cloning and as molecular probes. In nature, oligonucleotides are usually found as small RNA molecules that function in the regulation of gene expression, or are degradation intermediates derived from the breakdown of larger nucleic acid molecules.

Aptamers are oligomers of artificial ssDNA, RNA, XNA, or peptide that bind a specific target molecule, or family of target molecules. They exhibit a range of affinities, with variable levels of off-target binding and are sometimes classified as chemical antibodies. Aptamers and antibodies can be used in many of the same applications, but the nucleic acid-based structure of aptamers, which are mostly oligonucleotides, is very different from the amino acid-based structure of antibodies, which are proteins. This difference can make aptamers a better choice than antibodies for some purposes.

Nucleoproteins are proteins conjugated with nucleic acids. Typical nucleoproteins include ribosomes, nucleosomes and viral nucleocapsid proteins.
Steven Albert Benner is an American chemist. He has been a professor at Harvard University, ETH Zurich, and most recently at the University of Florida, where he was the V.T. & Louise Jackson Distinguished Professor of Chemistry. In 2005, he founded The Westheimer Institute of Science and Technology (TWIST) and the Foundation For Applied Molecular Evolution. Benner has also founded the companies EraGen Biosciences and Firebird BioMolecular Sciences LLC.
Nucleic acid structure prediction is a computational method to determine secondary and tertiary nucleic acid structure from its sequence. Secondary structure can be predicted from one or several nucleic acid sequences. Tertiary structure can be predicted from the sequence, or by comparative modeling.

Molecular biophysics is a rapidly evolving interdisciplinary area of research that combines concepts in physics, chemistry, engineering, mathematics and biology. It seeks to understand biomolecular systems and explain biological function in terms of molecular structure, structural organization, and dynamic behaviour at various levels of complexity. This discipline covers topics such as the measurement of molecular forces, molecular associations, allosteric interactions, Brownian motion, and cable theory. Additional areas of study can be found on Outline of Biophysics. The discipline has required development of specialized equipment and procedures capable of imaging and manipulating minute living structures, as well as novel experimental approaches.
Adriaan "Ad" Bax is a Dutch-American molecular biophysicist. He was born in the Netherlands and is the Chief of the Section on Biophysical NMR Spectroscopy at the National Institutes of Health. He is known for his work on the methodology of biomolecular NMR spectroscopy. He is a corresponding member of the Royal Netherlands Academy of Arts and Sciences, a member of the National Academy of Sciences, a fellow of the American Academy of Arts and Sciences, and a Foreign Member of the Royal Society.
Experimental approaches of determining the structure of nucleic acids, such as RNA and DNA, can be largely classified into biophysical and biochemical methods. Biophysical methods use the fundamental physical properties of molecules for structure determination, including X-ray crystallography, NMR and cryo-EM. Biochemical methods exploit the chemical properties of nucleic acids using specific reagents and conditions to assay the structure of nucleic acids. Such methods may involve chemical probing with specific reagents, or rely on native or analogue chemistry. Different experimental approaches have unique merits and are suitable for different experimental purposes.
This is a list of notable computer programs that are used for nucleic acids simulations.
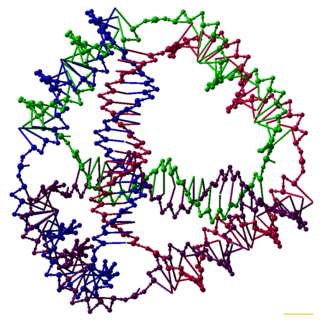
DNA nanotechnology is the design and manufacture of artificial nucleic acid structures for technological uses. In this field, nucleic acids are used as non-biological engineering materials for nanotechnology rather than as the carriers of genetic information in living cells. Researchers in the field have created static structures such as two- and three-dimensional crystal lattices, nanotubes, polyhedra, and arbitrary shapes, and functional devices such as molecular machines and DNA computers. The field is beginning to be used as a tool to solve basic science problems in structural biology and biophysics, including applications in X-ray crystallography and nuclear magnetic resonance spectroscopy of proteins to determine structures. Potential applications in molecular scale electronics and nanomedicine are also being investigated.

Nucleic acid secondary structure is the basepairing interactions within a single nucleic acid polymer or between two polymers. It can be represented as a list of bases which are paired in a nucleic acid molecule. The secondary structures of biological DNAs and RNAs tend to be different: biological DNA mostly exists as fully base paired double helices, while biological RNA is single stranded and often forms complex and intricate base-pairing interactions due to its increased ability to form hydrogen bonds stemming from the extra hydroxyl group in the ribose sugar.
Nucleic acid NMR is the use of nuclear magnetic resonance spectroscopy to obtain information about the structure and dynamics of nucleic acid molecules, such as DNA or RNA. It is useful for molecules of up to 100 nucleotides, and as of 2003, nearly half of all known RNA structures had been determined by NMR spectroscopy.
The 3' splice site of the influenza A virus segment 7 pre-mRNA can adopt two different types of RNA structure: a pseudoknot and a hairpin. This conformational switch is proposed to play a role in RNA alternative splicing and may influence the production of M1 and M2 proteins produced by splicing of this pre-mRNA.
The ViennaRNA Package is software, a set of standalone programs and libraries used for predicting and analysing RNA nucleic acid secondary structures. The source code for the package is released as free and open-source software and compiled binaries are available for the operating systems Linux, macOS, and Windows. The original paper has been cited over 2,000 times.
Non-canonical base pairs are planar hydrogen bonded pairs of nucleobases, having hydrogen bonding patterns which differ from the patterns observed in Watson-Crick base pairs, as in the classic double helical DNA. The structures of polynucleotide strands of both DNA and RNA molecules can be understood in terms of sugar-phosphate backbones consisting of phosphodiester-linked D 2’ deoxyribofuranose sugar moieties, with purine or pyrimidine nucleobases covalently linked to them. Here, the N9 atoms of the purines, guanine and adenine, and the N1 atoms of the pyrimidines, cytosine and thymine, respectively, form glycosidic linkages with the C1’ atom of the sugars. These nucleobases can be schematically represented as triangles with one of their vertices linked to the sugar, and the three sides accounting for three edges through which they can form hydrogen bonds with other moieties, including with other nucleobases. The side opposite to the sugar linked vertex is traditionally called the Watson-Crick edge, since they are involved in forming the Watson-Crick base pairs which constitute building blocks of double helical DNA. The two sides adjacent to the sugar-linked vertex are referred to, respectively, as the Sugar and Hoogsteen edges.

N1-Methylpseudouridine is a natural archaeal tRNA component, and "hypermodified" pyrimidine nucleoside used in biochemistry and molecular biology for in vitro transcription and is found in the SARS-CoV-2 mRNA vaccines tozinameran (Pfizer–BioNTech) and elasomeran (Moderna).

Sfold is a software program developed to predict probable RNA secondary structures through structure ensemble sampling and centroid predictions with a focus on assessment of RNA target accessibility, for major applications to the rational design of siRNAs in the suppression of gene expressions, and to the identification of targets for regulatory RNAs particularly microRNAs.