Related Research Articles
Biostatistics is a branch of statistics that applies statistical methods to a wide range of topics in biology. It encompasses the design of biological experiments, the collection and analysis of data from those experiments and the interpretation of the results.

The design of experiments, also known as experiment design or experimental design, is the design of any task that aims to describe and explain the variation of information under conditions that are hypothesized to reflect the variation. The term is generally associated with experiments in which the design introduces conditions that directly affect the variation, but may also refer to the design of quasi-experiments, in which natural conditions that influence the variation are selected for observation.
Evidence-based medicine (EBM) is "the conscientious, explicit and judicious use of current best evidence in making decisions about the care of individual patients." The aim of EBM is to integrate the experience of the clinician, the values of the patient, and the best available scientific information to guide decision-making about clinical management. The term was originally used to describe an approach to teaching the practice of medicine and improving decisions by individual physicians about individual patients.
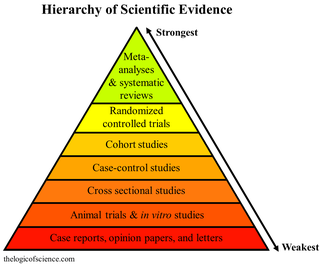
A meta-analysis is a statistical analysis that combines the results of multiple scientific studies. Meta-analyses can be performed when there are multiple scientific studies addressing the same question, with each individual study reporting measurements that are expected to have some degree of error. The aim then is to use approaches from statistics to derive a pooled estimate closest to the unknown common truth based on how this error is perceived. It is thus a basic methodology of metascience. Meta-analytic results are considered the most trustworthy source of evidence by the evidence-based medicine literature.

A randomized controlled trial is a form of scientific experiment used to control factors not under direct experimental control. Examples of RCTs are clinical trials that compare the effects of drugs, surgical techniques, medical devices, diagnostic procedures or other medical treatments.
A gastrointestinal cocktail,, is a mixture of medications used to treat symptoms of dyspepsia The GI cocktail generally contains a mixture of viscous lidocaine, an antacid, and an anticholinergic. The GI cocktail is commonly prescribed in the hospital or emergency department, and has been used to help distinguish chest pain as either gastrointestinal or cardiac. While it has been widely used in the treatment of dyspepsia, studies have suggested that the GI cocktail is only as effective as antacids alone.

In causal inference, a confounder is a variable that influences both the dependent variable and independent variable, causing a spurious association. Confounding is a causal concept, and as such, cannot be described in terms of correlations or associations. The existence of confounders is an important quantitative explanation why correlation does not imply causation. Some notations are explicitly designed to identify the existence, possible existence, or non-existence of confounders in causal relationships between elements of a system.
In statistics, sequential analysis or sequential hypothesis testing is statistical analysis where the sample size is not fixed in advance. Instead data are evaluated as they are collected, and further sampling is stopped in accordance with a pre-defined stopping rule as soon as significant results are observed. Thus a conclusion may sometimes be reached at a much earlier stage than would be possible with more classical hypothesis testing or estimation, at consequently lower financial and/or human cost.
A hierarchy of evidence, comprising levels of evidence (LOEs), that is, evidence levels (ELs), is a heuristic used to rank the relative strength of results obtained from experimental research, especially medical research. There is broad agreement on the relative strength of large-scale, epidemiological studies. More than 80 different hierarchies have been proposed for assessing medical evidence. The design of the study and the endpoints measured affect the strength of the evidence. In clinical research, the best evidence for treatment efficacy is mainly from meta-analyses of randomized controlled trials (RCTs). Systematic reviews of completed, high-quality randomized controlled trials – such as those published by the Cochrane Collaboration – rank the same as systematic review of completed high-quality observational studies in regard to the study of side effects. Evidence hierarchies are often applied in evidence-based practices and are integral to evidence-based medicine (EBM).

In science, randomized experiments are the experiments that allow the greatest reliability and validity of statistical estimates of treatment effects. Randomization-based inference is especially important in experimental design and in survey sampling.
Repeated measures design is a research design that involves multiple measures of the same variable taken on the same or matched subjects either under different conditions or over two or more time periods. For instance, repeated measurements are collected in a longitudinal study in which change over time is assessed.

James M. Robins is an epidemiologist and biostatistician best known for advancing methods for drawing causal inferences from complex observational studies and randomized trials, particularly those in which the treatment varies with time. He is the 2013 recipient of the Nathan Mantel Award for lifetime achievement in statistics and epidemiology, and a recipient of the 2022 Rousseeuw Prize in Statistics, jointly with Miguel Hernán, Eric Tchetgen-Tchetgen, Andrea Rotnitzky and Thomas Richardson.
An N of 1 trial (N=1) is a clinical trial in which a single patient is the entire trial, a single case study. A trial in which random allocation can be used to determine the order in which an experimental and a control intervention are given to a patient is an N of 1 randomized controlled trial. The order of experimental and control interventions can also be fixed by the researcher.
Clinical behavior analysis is the clinical application of behavior analysis (ABA). CBA represents a movement in behavior therapy away from methodological behaviorism and back toward radical behaviorism and the use of functional analytic models of verbal behavior—particularly, relational frame theory (RFT).
Principal stratification is a statistical technique used in causal inference when adjusting results for post-treatment covariates. The idea is to identify underlying strata and then compute causal effects only within strata. It is a generalization of the local average treatment effect (LATE) in the sense of presenting applications besides all-or-none compliance. The LATE method, which was independently developed by Imbens and Angrist (1994) and Baker and Lindeman (1994) also included the key exclusion restriction and monotonicity assumptions for identifiability. For the history of early developments see Baker, Kramer, Lindeman.
Marvin Zelen was Professor Emeritus of Biostatistics in the Department of Biostatistics at the Harvard T.H. Chan School of Public Health (HSPH), and Lemuel Shattuck Research Professor of Statistical Science. During the 1980s, Zelen chaired HSPH's Department of Biostatistics. Among colleagues in the field of statistics, he was widely known as a leader who shaped the discipline of biostatistics. He "transformed clinical trial research into a statistically sophisticated branch of medical research."
Erica Eleanor Margret Moodie is a Canadian biostatistician known for her work on dynamic treatment regimes. She is Canada Research Chair and Professor in the Department of Epidemiology, Biostatistics, and Occupational Health at McGill University.

Roderick Joseph Alexander Little is an academic statistician, whose main research contributions lie in the statistical analysis of data with missing values and the analysis of complex sample survey data. Little is Richard D. Remington Distinguished University Professor of Biostatistics in the Department of Biostatistics at the University of Michigan, where he also holds academic appointments in the Department of Statistics and the Institute for Social Research.
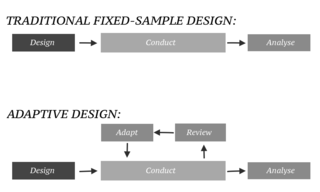
In an adaptive design of a clinical trial, the parameters and conduct of the trial for a candidate drug or vaccine may be changed based on an interim analysis. Adaptive design typically involves advanced statistics to interpret a clinical trial endpoint. This is in contrast to traditional single-arm clinical trials or randomized clinical trials (RCTs) that are static in their protocol and do not modify any parameters until the trial is completed. The adaptation process takes place at certain points in the trial, prescribed in the trial protocol. Importantly, this trial protocol is set before the trial begins with the adaptation schedule and processes specified. Adaptions may include modifications to: dosage, sample size, drug undergoing trial, patient selection criteria and/or "cocktail" mix. The PANDA provides not only a summary of different adaptive designs, but also comprehensive information on adaptive design planning, conduct, analysis and reporting.
Robert L. Strawderman is an academic biostatistician and researcher who holds the Donald M. Foster, MD Distinguished Professorship in Biostatistics at the University of Rochester. He has served as chair of the Department of Biostatistics and Computational Biology since 2012. Strawderman's principal research interests include semiparametric methods for missing and censored data and statistical learning methods for risk and outcome prediction. Contributions in numerous other areas include inference and variable selection in the areas of dynamic treatment regimes and causal inference in mediation analysis and for recurrent events.
References
- ↑ Lei, H.; Nahum-Shani, I.; Lynch, K.; Oslin, D.; Murphy, S. A. (2012), "A "SMART" design for building individualized treatment sequences", Annual Review of Clinical Psychology, 8: 21–48, doi:10.1146/annurev-clinpsy-032511-143152, PMC 3887122 , PMID 22224838
- ↑ Wagner E. H.; Austin B. T.; Davis C.; Hindmarsh M.; Schaefer J.; Bonomi A. (2001). "Improving Chronic Illness Care: Translating Evidence Into Action". Health Affairs. 20 (6): 64–78. doi:10.1377/hlthaff.20.6.64. PMID 11816692.