Related Research Articles

Dysprosium is a chemical element; it has symbol Dy and atomic number 66. It is a rare-earth element in the lanthanide series with a metallic silver luster. Dysprosium is never found in nature as a free element, though, like other lanthanides, it is found in various minerals, such as xenotime. Naturally occurring dysprosium is composed of seven isotopes, the most abundant of which is 164Dy.

Holmium is a chemical element; it has symbol Ho and atomic number 67. It is a rare-earth element and the eleventh member of the lanthanide series. It is a relatively soft, silvery, fairly corrosion-resistant and malleable metal. Like many other lanthanides, holmium is too reactive to be found in native form, as pure holmium slowly forms a yellowish oxide coating when exposed to air. When isolated, holmium is relatively stable in dry air at room temperature. However, it reacts with water and corrodes readily, and also burns in air when heated.

Solder is a fusible metal alloy used to create a permanent bond between metal workpieces. Solder is melted in order to wet the parts of the joint, where it adheres to and connects the pieces after cooling. Metals or alloys suitable for use as solder should have a lower melting point than the pieces to be joined. The solder should also be resistant to oxidative and corrosive effects that would degrade the joint over time. Solder used in making electrical connections also needs to have favorable electrical characteristics.

A neodymium magnet (also known as NdFeB, NIB or Neo magnet) is a permanent magnet made from an alloy of neodymium, iron, and boron to form the Nd2Fe14B tetragonal crystalline structure. They are the most widely used type of rare-earth magnet.

In crystallography, the cubiccrystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.

An intermetallic is a type of metallic alloy that forms an ordered solid-state compound between two or more metallic elements. Intermetallics are generally hard and brittle, with good high-temperature mechanical properties. They can be classified as stoichiometric or nonstoichiometic intermetallic compounds.

The A15 phases (also known as β-W or Cr3Si structure types) are series of intermetallic compounds with the chemical formula A3B (where A is a transition metal and B can be any element) and a specific structure. The A15 phase is also one of the members in the Frank–Kasper phases family. Many of these compounds have superconductivity at around 20 K (−253 °C; −424 °F), which is comparatively high, and remain superconductive in magnetic fields of tens of teslas (hundreds of kilogauss). This kind of superconductivity (Type-II superconductivity) is an important area of study as it has several practical applications.
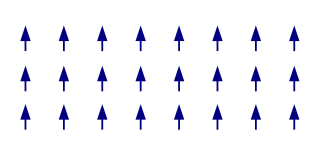
The term magnetic structure of a material pertains to the ordered arrangement of magnetic spins, typically within an ordered crystallographic lattice. Its study is a branch of solid-state physics.
An orthoferrite is any of a class of chemical compounds with the formula RFeO3, where R is one or more rare-earth elements. Orthoferrites have an orthorhombic crystal structure with a space group Pbnm and most are weakly ferromagnetic. At the Néel temperature the subsystem of iron ions orders into a slightly canted antiferromagnetic structure with antiferromagnetic moment G and a weak ferromagnetic moment F. The rare-earth ion subsystem acquires magnetization m due to an interaction with the iron subsystem.

Plumbide is an anion of lead atoms. There are three plumbide anions, written as Pb−, Pb2− and Pb4− with 3 oxidation states, -1, -2 and -4, respectively. A plumbide can refer to one of two things: an intermetallic compound that contains lead, or a Zintl phase compound with lead as the anion.
A stannide can refer to an intermetallic compound containing tin combined with one or more other metals; an anion consisting solely of tin atoms or a compound containing such an anion, or, in the field of organometallic chemistry an ionic compound containing an organotin anion
High-entropy alloys (HEAs) are alloys that are formed by mixing equal or relatively large proportions of (usually) five or more elements. Prior to the synthesis of these substances, typical metal alloys comprised one or two major components with smaller amounts of other elements. For example, additional elements can be added to iron to improve its properties, thereby creating an iron-based alloy, but typically in fairly low proportions, such as the proportions of carbon, manganese, and others in various steels. Hence, high-entropy alloys are a novel class of materials. The term "high-entropy alloys" was coined by Taiwanese scientist Jien-Wei Yeh because the entropy increase of mixing is substantially higher when there is a larger number of elements in the mix, and their proportions are more nearly equal. Some alternative names, such as multi-component alloys, compositionally complex alloys and multi-principal-element alloys are also suggested by other researchers.

A holmium–magnesium–zinc (Ho–Mg–Zn) quasicrystal is a quasicrystal made of an alloy of the three metals holmium, magnesium and zinc that has the shape of a regular dodecahedron, a Platonic solid with 12 five-sided faces. Unlike the similar pyritohedron shape of some cubic-system crystals such as pyrite, this quasicrystal has faces that are true regular pentagons.
Dysprosium(II) chloride (DyCl2), also known as dysprosium dichloride, is an ionic chemical compound of dysprosium and chlorine. This salt is a reduced compound, as the normal oxidation state of dysprosium in dysprosium compounds is +3.
Silicide carbides or carbide silicides are compounds containing anions composed of silicide (Si4−) and carbide (C4−) or clusters therof. They can be considered as mixed anion compounds or intermetallic compounds, as silicon could be considered as a semimetal.
Dysprosium phosphide is an inorganic compound of dysprosium and phosphorus with the chemical formula DyP.

Terbium compounds are compounds formed by the lanthanide metal terbium (Tb). Terbium generally exhibits the +3 oxidation state in these compounds, such as in TbCl3, Tb(NO3)3 and Tb(CH3COO)3. Compounds with terbium in the +4 oxidation state are also known, such as TbO2 and BaTbF6. Terbium can also form compounds in the 0, +1 and +2 oxidation states.

Dysprosium(III) phosphate is an inorganic compound with the chemical formula DyPO4.
Dysprosium(III) telluride is an inorganic compound, one of the tellurides of dysprosium, with the chemical formula Dy2Te3, where Dy is in the +3 oxidation state.
Yttrium and tin form several yttrium stannide intermetallic compounds.
References
- ↑ Okamoto, H. (2005-04-01). "Dy-Sn (Dysprosium-Tin)". Journal of Phase Equilibria & Diffusion. 26 (2): 200–202. doi:10.1361/15477030523247.
- ↑ Colinet, C.; Pasturel, A.; Percheron-Guégan, A.; Achard, J.C. (October 1984). "Experimental and calculated enthalpies of formation of rare earth-tin alloys". Journal of the Less Common Metals. 102 (2): 173. doi:10.1016/0022-5088(84)90313-8.
- ↑ Venturini, G.; Lemoine, P.; Malaman, B.; Ouladdiaf, B. (September 2010). "Magnetic structures of orthorhombic rare earth stannides LSn2 (L=Tb–Tm)". Journal of Alloys and Compounds. 505 (2): 404–415. doi:10.1016/j.jallcom.2010.06.089.