Related Research Articles

Cancer immunotherapy is the stimulation of the immune system to treat cancer, improving on the immune system's natural ability to fight the disease. It is an application of the fundamental research of cancer immunology and a growing subspecialty of oncology.
Deoxyribozymes, also called DNA enzymes, DNAzymes, or catalytic DNA, are DNA oligonucleotides that are capable of performing a specific chemical reaction, often but not always catalytic. This is similar to the action of other biological enzymes, such as proteins or ribozymes . However, in contrast to the abundance of protein enzymes in biological systems and the discovery of biological ribozymes in the 1980s, there is only little evidence for naturally occurring deoxyribozymes. Deoxyribozymes should not be confused with DNA aptamers which are oligonucleotides that selectively bind a target ligand, but do not catalyze a subsequent chemical reaction.

Targeted therapy or molecularly targeted therapy is one of the major modalities of medical treatment (pharmacotherapy) for cancer, others being hormonal therapy and cytotoxic chemotherapy. As a form of molecular medicine, targeted therapy blocks the growth of cancer cells by interfering with specific targeted molecules needed for carcinogenesis and tumor growth, rather than by simply interfering with all rapidly dividing cells. Because most agents for targeted therapy are biopharmaceuticals, the term biologic therapy is sometimes synonymous with targeted therapy when used in the context of cancer therapy. However, the modalities can be combined; antibody-drug conjugates combine biologic and cytotoxic mechanisms into one targeted therapy.

Sorafenib, sold under the brand name Nexavar, is a kinase inhibitor drug approved for the treatment of primary kidney cancer, advanced primary liver cancer, FLT3-ITD positive AML and radioactive iodine resistant advanced thyroid carcinoma.

Sunitinib, sold under the brand name Sutent, is a medication used to treat cancer. It is a small-molecule, multi-targeted receptor tyrosine kinase (RTK) inhibitor that was approved by the FDA for the treatment of renal cell carcinoma (RCC) and imatinib-resistant gastrointestinal stromal tumor (GIST) on January 26, 2006. Sunitinib was the first cancer drug simultaneously approved for two different indications.
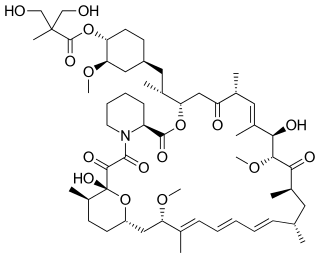
Temsirolimus, sold under the brand name Torisel, is an intravenous drug for the treatment of renal cell carcinoma (RCC), developed by Wyeth Pharmaceuticals and approved by the U.S. Food and Drug Administration (FDA) in May 2007, and was also approved by the European Medicines Agency (EMA) in November 2007. It is a derivative and prodrug of sirolimus.

72 kDa type IV collagenase also known as matrix metalloproteinase-2 (MMP-2) and gelatinase A is an enzyme that in humans is encoded by the MMP2 gene. The MMP2 gene is located on chromosome 16 at position 12.2.
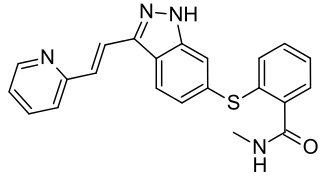
Axitinib, sold under the brand name Inlyta, is a small molecule tyrosine kinase inhibitor developed by Pfizer. It has been shown to significantly inhibit growth of breast cancer in animal (xenograft) models and has shown partial responses in clinical trials with renal cell carcinoma (RCC) and several other tumour types.
Cancer immunoprevention is the prevention of cancer onset with immunological means such as vaccines, immunostimulators or antibodies. Cancer immunoprevention is conceptually different from cancer immunotherapy, which aims at stimulating immunity in patients only after tumor onset, however the same immunological means can be used both in immunoprevention and in immunotherapy.
Metalloprotease inhibitors are cellular inhibitors of the Matrix metalloproteinases (MMPs). MMPs belong to a family of zinc-dependent neutral endopeptidases. These enzymes have the ability to break down connective tissue. The expression of MMPs is increased in various pathological conditions like inflammatory conditions, metabolic bone disease, to cancer invasion, metastasis and angiogenesis. Examples of diseases are periodontitis, hepatitis, glomerulonephritis, atherosclerosis, emphysema, asthma, autoimmune disorders of skin and dermal photoaging, rheumatoid arthritis, osteoarthritis, multiple sclerosis, Alzheimer's disease, chronic ulcerations, uterine involution, corneal epithelial defects, bone resorption and tumor progression and metastasis. Due to the role of MMPs in pathological conditions, inhibitors of MMPs may have therapeutic potential. Several other proteins have similar inhibitory effects, however none as effective. They might have other biological activities which have yet been fully characterised.

In molecular biology miR-203 is a short non-coding RNA molecule. MicroRNAs function to regulate the expression levels of other genes by several mechanisms, such as translational repression and Argonaute-catalyzed messenger RNA cleavage. miR-203 has been identified as a skin-specific microRNA, and it forms an expression gradient that defines the boundary between proliferative epidermal basal progenitors and terminally differentiating suprabasal cells. It has also been found upregulated in psoriasis and differentially expressed in some types of cancer.
JX-594 is an oncolytic virus is designed to target and destroy cancer cells. It is also known as Pexa-Vec, INN pexastimogene devacirepvec) and was constructed in Dr. Edmund Lattime's lab at Thomas Jefferson University, tested in clinical trials on melanoma patients, and licensed and further developed by SillaJen.
Tumor-associated macrophages (TAMs) are a class of immune cells present in high numbers in the microenvironment of solid tumors. They are heavily involved in cancer-related inflammation. Macrophages are known to originate from bone marrow-derived blood monocytes or yolk sac progenitors, but the exact origin of TAMs in human tumors remains to be elucidated. The composition of monocyte-derived macrophages and tissue-resident macrophages in the tumor microenvironment depends on the tumor type, stage, size, and location, thus it has been proposed that TAM identity and heterogeneity is the outcome of interactions between tumor-derived, tissue-specific, and developmental signals.

Pembrolizumab, sold under the brand name Keytruda, is a humanized antibody used in cancer immunotherapy that treats melanoma, lung cancer, head and neck cancer, Hodgkin lymphoma, stomach cancer, cervical cancer, and certain types of breast cancer. It is given by slow injection into a vein.
Levon Michael Khachigian is an Australian medical research scientist notable for his work in vascular cell and molecular biology. He is a Professor in the Faculty of Medicine at the University of New South Wales.

Sonidegib (INN), sold under the brand name Odomzo, is a medication used to treat cancer.
Avelumab, sold under the brand name Bavencio, is a fully human monoclonal antibody medication for the treatment of Merkel cell carcinoma, urothelial carcinoma, and renal cell carcinoma.

PD-1 inhibitors and PD-L1 inhibitors are a group of checkpoint inhibitor anticancer drugs that block the activity of PD-1 and PDL1 immune checkpoint proteins present on the surface of cells. Immune checkpoint inhibitors are emerging as a front-line treatment for several types of cancer.
Cemiplimab, sold under the brand name Libtayo, is a monoclonal antibody medication for the treatment of squamous cell skin cancer. Cemiplimab belongs to a class of drugs that binds to the programmed death receptor-1 (PD-1), blocking the PD-1/PD-L1 pathway.

Sotorasib, sold under the brand names Lumakras and Lumykras, is an anti-cancer medication used to treat non-small-cell lung cancer. It targets a specific mutation, G12C, in the protein K-Ras encoded by gene KRAS which is responsible for various forms of cancer. Sotorasib is an inhibitor of the RAS GTPase family.
References
- ↑ Khachigian, L.M. et al (2002) c-Jun regulates vascular smooth muscle cell growth and neointima formation after arterial injury. Inhibition by a novel DNA enzyme targeting c-Jun. J Biol Chem. 277(25):22985-91.
- ↑ Kahan-Hanum, M. et al (2013) A library of programmable DNAzymes that operate in a cellular environment. Sci Rep. 3:1535.
- ↑ Zhang, G. et al (2006) Squamous cell carcinoma growth in mice and in culture is regulated by c-Jun and its control of matrix metalloproteinase-2 and -9 expression. Oncogene. 25(55): 7260-6.
- 1 2 Tan, M.L. et al (2010) Direct anti-metastatic efficacy by the DNA enzyme Dz13 and downregulated MMP-2, MMP-9 and MT1-MMP in tumours. Cancer Cell Int. 10: 9.
- 1 2 Xie, J. et al (2014) Regulatory roles of c-jun in H5N1 influenza virus replication and host inflammation. Biochim Biophys Acta. 1842(12 Pt A):2479-88.
- ↑ Kynova, R. et al (2014) Enhancing nucleic acid delivery, insights from the cationic phospholipid carriers. Current Pharmaceutical Biotechnology 15(9), 806-13.
- ↑ Yang. J. et al (2019) Inhibition of proliferation and migration of tumor cells through phenylboronic acid-functionalized polyamidoamine-mediated delivery of a therapeutic DNAzyme Dz13. International Journal of Nanomedicine 14, 6371–6385.
- ↑ Zhang, Z. et al (2017) DNAzymes Dz13 target the c-jun possess antiviral activity against influenza A viruses. Microbial Pathogenesis 103, 155-161.
- ↑ Kim, M.-G. et al (2015) Biomimetic DNA nanoballs for oligonucleotide delivery. Biomaterials 62, 155-163.
- ↑ Marquardt, K. et al (2015) Development of a protective dermal drug delivery system for therapeutic DNAzymes. International Journal of Pharmaceutics 479, 150-158.
- ↑ Rivory, L. et al (2006) The DNAzymes Rs6, Dz13, and DzF have potent biologic effects independent of catalytic activity. Oligonucleotides 16, 297–312.
- ↑ Goodchild, A. et al (2007) Cytotoxic G-rich oligode-oxynucleotides: putative protein targets and required sequence motif. Nucleic Acids Res. 35, 4562–4572.
- ↑ Gozar, M.M. et al (2008) Dz13, a DNAzyme Targeting c-jun, Induces Off-Target Cytotoxicity in Endothelial Cells with Features of Nonapoptotic Programmed Cell Death. Oligonucleotides 18(3), 257-268.
- ↑ Fahmy, R. et al (2006) Suppression of vascular permeability and inflammation by targeting of the transcription factor c-Jun. Nat Biotechnol. 24(7): 856-863.
- ↑ Cai, H. et al (2012) DNAzyme targeting c-jun suppresses skin cancer growth. Science Transl Med. 4(139): 139ra82.
- ↑ Australian Cancer Trials (2010) A phase I study of the Dz13 drug targeting the c-Jun gene in subjects with skin cancer (nodular basal cell carcinoma). .
- ↑ Cho, E.A. et al (2013) Safety and tolerability of an intratumorally injected DNAzyme, Dz13, in patients with nodular basal-cell carcinoma: a phase 1 first-in-human trial (DISCOVER). Lancet. 381(9880):1835-43
- 1 2 Cao, Y. et al (2014) Therapeutic evaluation of Epstein-Barr virus-encoded latent membrane protein-1 targeted DNAzyme for treating of nasopharyngeal carcinomas.
- 1 2 Krug, N. et al (2015) Allergen-induced asthmatic responses modified by a GATA3-specific DNAzyme. N Engl J Med. 372(21):1987-95.
- ↑ Scott, S. "Trials of skin cancer drug DZ13 suspended pending investigation at UNSW". Australian Broadcasting Corporation . Retrieved 9 April 2019.
- ↑ Scott, S. "University of NSW defends handling of investigation into prominent scientist Levon Khachigian". Australian Broadcasting Corporation . Retrieved 9 April 2019.
- ↑ "UNSW clears Levon Khachigian of all allegations of research misconduct". The Australian
- ↑ "UNSW skin cancer researcher Levon Khachigian hit with string of retractions - ABC News".