Related Research Articles

A steroid is a biologically active organic compound with four rings arranged in a specific molecular configuration. Steroids have two principal biological functions: as important components of cell membranes that alter membrane fluidity; and as signaling molecules. Hundreds of steroids are found in plants, animals and fungi. All steroids are manufactured in cells from the sterols lanosterol (opisthokonts) or cycloartenol (plants). Lanosterol and cycloartenol are derived from the cyclization of the triterpene squalene.

Ergosterol (ergosta-5,7,22-trien-3β-ol) is a sterol found in cell membranes of fungi and protozoa, serving many of the same functions that cholesterol serves in animal cells. Because many fungi and protozoa cannot survive without ergosterol, the enzymes that synthesize it have become important targets for drug discovery. In human nutrition, ergosterol is a provitamin form of vitamin D2; exposure to ultraviolet (UV) light causes a chemical reaction that produces vitamin D2.
Miconazole, sold under the brand name Monistat among others, is an antifungal medication used to treat ring worm, pityriasis versicolor, and yeast infections of the skin or vagina. It is used for ring worm of the body, groin, and feet. It is applied to the skin or vagina as a cream or ointment.

Sterol is an organic compound with formula C
17H
28O, whose molecule is derived from that of gonane by replacement of a hydrogen atom in position 3 by a hydroxyl group. It is therefore an alcohol of gonane. More generally, any compounds that contain the gonane structure, additional functional groups, and/or modified ring systems derived from gonane are called steroids. Therefore, sterols are a subgroup of the steroids. They occur naturally in most eukaryotes, including plants, animals, and fungi, and can also be produced by some bacteria. The most familiar type of animal sterol is cholesterol, which is vital to cell membrane structure, and functions as a precursor to fat-soluble vitamins and steroid hormones.

7-Dehydrocholesterol reductase, also known as DHCR7, is a protein that in humans is encoded by the DHCR7 gene.
In enzymology, a Delta24(241)-sterol reductase (EC 1.3.1.71) is an enzyme that catalyzes the chemical reaction
In enzymology, a Delta24-sterol reductase (EC 1.3.1.72) is an enzyme that catalyzes the chemical reaction

In enzymology, a sterol 14-demethylase (EC 1.14.13.70) is an enzyme of the Cytochrome P450 (CYP) superfamily. It is any member of the CYP51 family. It catalyzes a chemical reaction such as:

Lathosterol oxidase is a Δ7-sterol 5(6)-desaturase enzyme that in humans is encoded by the SC5D gene.

Delta(14)-sterol reductase is an enzyme that in humans is encoded by the TM7SF2 gene.
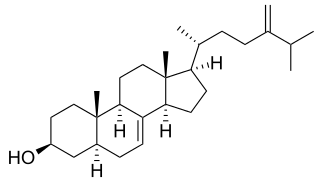
Episterol is a sterol involved in the biosynthesis of steroids. Episterol is converted from 24-methylenelophenol. Episterol is converted to 5-dehydroepisterol by ERG3, the C-5 sterol desaturase in the yeast. Episterol is also known to be a precursor to ergosterol.
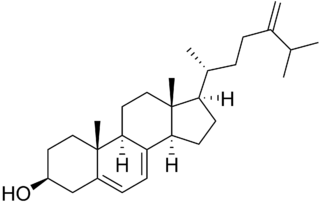
5-Dehydroepisterol is a sterol and an intermediate in steroid biosynthesis, particularly synthesis of brassinosteroids. It is formed from episterol through action of ERG3, the C-5 sterol desaturase in the yeast and is then converted into 24-methylenecholesterol by 7-dehydrocholesterol reductase.

Ergosterol peroxide (5α,8α-epidioxy-22E-ergosta-6,22-dien-3β-ol) is a steroid derivative. It has been isolated from a variety of fungi, yeast, lichens and sponges, and has been reported to exhibit immunosuppressive, anti-inflammatory, antiviral, trypanocidal and antitumor activities in vitro.

Cerevisterol (5α-ergosta-7,22-diene-3β,5,6β-triol) is a sterol. Originally described in the 1930s from the yeast Saccharomyces cerevisiae, it has since been found in several other fungi and, recently, in deep water coral. Cerevisterol has some in vitro bioactive properties, including cytotoxicity to some mammalian cell lines.

C-5 sterol desaturase is an enzyme that is highly conserved among eukaryotes and catalyzes the dehydrogenation of a C-5(6) bond in a sterol intermediate compound as a step in the biosynthesis of major sterols. The precise structure of the enzyme's substrate varies by species. For example, the human C-5 sterol desaturase oxidizes lathosterol, while its ortholog ERG3 in the yeast Saccharomyces cerevisiae oxidizes episterol.

4α-Methylfecosterol is a metabolic intermediate of sterols made by certain fungis, can be converted to 24-Methylenelophenol by enzyme HYD1, or undergo 4-demethylation to fecosterol.
ERG11 or Sterol 14-demethylase is a fungal cytochrome P450 enzyme originally from Saccharomyces cerevisiae, belongs to family CYP51, with the CYP Symbol CYP51F1. ERG11 catalyzes the C14-demethylation of lanosterol to 4,4'-dimethyl cholesta-8,14,24-triene-3-beta-ol which is the first step of biosynthesis of the zymosterol, zymosterol will be further converted into Ergosterol.

ERG3 or sterol C-5 desaturase is a fungal enzyme originally from Saccharomyces cerevisiae, the human ortholog of ERG3 is SC5D. ERG3 localizes to both the endoplasmic reticulum and vesicles, catalyzes the C5(6)-dehydrogenation of episterol to 5-dehydroepisterol, 5-Dehydroepisterol will be further converted into ergosterol.
ERG5 or Sterol 22-desaturase is a cytochrome P450 enzyme in the ergosterol biosynthesis pathway of fungi Saccharomyces cerevisiae, with the CYP Symbol CYP61A1. CYP61A1 is one of only three P450 enzyme found in baker's yeast, the other two are CYP51F1 and CYP56A1. The ortholog in Schizosaccharomyces pombe, was named CYP61A3 for historical reasons, and is only one of two P450 enzyme found with CYP51F1. ERG5 catalyzes the C22-C23 double bond formation on the sterol side chain of ergostatrienol to convert it into ergostatetraenol, then the C24 double bond of ergostatetrenol will be hydrogenation reduced into ergosterol by ERG4.

24-Methylenelophenol, or Gramisterol, also called 4α-Methyl-5α-ergosta-7,24(28)-dien-3β-ol is a Metabolic intermediate of sterol biosynthesis of plants and fungis, can be converted from 4α-Methylfecosterol by enzyme HYD1 and converted to (Z)-24-ethylidenelophenol by 24-methylenesterol C-methyltransferase.