
Edward A. Bayer is an American-Israeli scientist.

Edward A. Bayer is an American-Israeli scientist.
He graduated with B.Sc. and M. Sc. in Zoology and Biology in University of Michigan and Wayne State University. He immigrated to Israel in 1971. He then graduated a Ph.D. in biophysics from the Weizmann Institute of Science, under the supervision of Meir Wilchek. Edward A. Bayer became a professor in the Weizmann Institute of Science in 2001 and published over 400 scientific papers. He is a member of the Scientific Advisory Board, of US-Department of Energy BioEnergy Science Center (BESC) since 2008 and of the Israel VATAT Planning and Budgeting Committee: National Subcommittee on "Energy & the Environment" and also serves as editor in several scientific journals.
Edward A. Bayer participated in the development of avidin-biotin technology [1] (together with Meir Wilchek) that contributed to the field of biorecognition and is intensively used in various biochemical applications, e.g. for affinity chromatography, affinity labeling, affinity therapy and many more. During his post-doctoral studies at Tel Aviv University, Edward A. Bayer co-discovered the Cellulosome [2] [3] (together with Raphael Lamed Professor at Tel Aviv University), an intricate multi-enzyme complex produced by many cellulolytic anaerobic microorganisms. [4] [5] In 1994, [6] [7] [8] Edward A. Bayer proposed an original concept for the construction of designer cellulosomes, based on the specific affinity between cohesin and dockerin modules from the same bacterial species and type.

These enzymatic complexes are composed of recombinant chimaeric scaffoldins and dockerin-containing enzyme hybrids and serve as nanomolecular tool for the characterization of cellulosomes. The use of designer cellulosome technology for the construction of a consolidated bioprocessing (CBP) organism to will combine enzyme production, cellulose hydrolysis, and fermentation into a single process for the conversion of lignocellulosic waste and biomass to valuables products such as sugars and biofuels has been demonstrated. [9] [10]

Biotin (also known as vitamin B7 or vitamin H) is one of the B vitamins. It is involved in a wide range of metabolic processes, both in humans and in other organisms, primarily related to the utilization of fats, carbohydrates, and amino acids. The name biotin, borrowed from the German Biotin, derives from the Ancient Greek word βίοτος (bíotos; 'life') and the suffix "-in" (a suffix used in chemistry usually to indicate 'forming'). Biotin appears as a white, needle-like crystalline solid.

Cellulase is any of several enzymes produced chiefly by fungi, bacteria, and protozoans that catalyze cellulolysis, the decomposition of cellulose and of some related polysaccharides:
In biochemistry, biotinylation is the process of covalently attaching biotin to a protein, nucleic acid or other molecule. Biotinylation is rapid, specific and is unlikely to disturb the natural function of the molecule due to the small size of biotin. Biotin binds to streptavidin and avidin with an extremely high affinity, fast on-rate, and high specificity, and these interactions are exploited in many areas of biotechnology to isolate biotinylated molecules of interest. Biotin-binding to streptavidin and avidin is resistant to extremes of heat, pH and proteolysis, making capture of biotinylated molecules possible in a wide variety of environments. Also, multiple biotin molecules can be conjugated to a protein of interest, which allows binding of multiple streptavidin, avidin or neutravidin protein molecules and increases the sensitivity of detection of the protein of interest. There is a large number of biotinylation reagents available that exploit the wide range of possible labelling methods. Due to the strong affinity between biotin and streptavidin, the purification of biotinylated proteins has been a widely used approach to identify protein-protein interactions and post-translational events such as ubiquitylation in molecular biology.
Cellulosic ethanol is ethanol produced from cellulose rather than from the plant's seeds or fruit. It can be produced from grasses, wood, algae, or other plants. It is generally discussed for use as a biofuel. The carbon dioxide that plants absorb as they grow offsets some of the carbon dioxide emitted when ethanol made from them is burned, so cellulosic ethanol fuel has the potential to have a lower carbon footprint than fossil fuels.
Affinity chromatography is a method of separating a biomolecule from a mixture, based on a highly specific macromolecular binding interaction between the biomolecule and another substance. The specific type of binding interaction depends on the biomolecule of interest; antigen and antibody, enzyme and substrate, receptor and ligand, or protein and nucleic acid binding interactions are frequently exploited for isolation of various biomolecules. Affinity chromatography is useful for its high selectivity and resolution of separation, compared to other chromatographic methods.

Streptavidin is a 52 kDa protein (tetramer) purified from the bacterium Streptomyces avidinii. Streptavidin homo-tetramers have an extraordinarily high affinity for biotin. With a dissociation constant (Kd) on the order of ≈10−14 mol/L, the binding of biotin to streptavidin is one of the strongest non-covalent interactions known in nature. Streptavidin is used extensively in molecular biology and bionanotechnology due to the streptavidin-biotin complex's resistance to organic solvents, denaturants, detergents, proteolytic enzymes, and extremes of temperature and pH.

Clostridium acetobutylicum, ATCC 824, is a commercially valuable bacterium sometimes called the "Weizmann Organism", after Jewish Russian-born biochemist Chaim Weizmann. A senior lecturer at the University of Manchester, England, he used them in 1916 as a bio-chemical tool to produce at the same time, jointly, acetone, ethanol, and n-butanol from starch. The method has been described since as the ABE process,, yielding 3 parts of acetone, 6 of n-butanol, and 1 of ethanol. Acetone was used in the important wartime task of casting cordite. The alcohols were used to produce vehicle fuels and synthetic rubber.
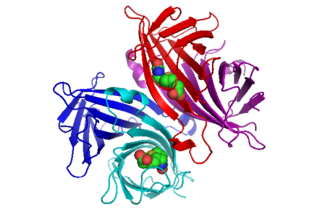
Avidin is a tetrameric biotin-binding protein produced in the oviducts of birds, reptiles and amphibians and deposited in the whites of their eggs. Dimeric members of the avidin family are also found in some bacteria. In chicken egg white, avidin makes up approximately 0.05% of total protein (approximately 1800 μg per egg). The tetrameric protein contains four identical subunits (homotetramer), each of which can bind to biotin (Vitamin B7, vitamin H) with a high degree of affinity and specificity. The dissociation constant of the avidin-biotin complex is measured to be KD ≈ 10−15 M, making it one of the strongest known non-covalent bonds.
Cellulosomes are multi-enzyme extracellular complexes. Cellulosomes are associated with the cell surface and mediate cell attachment to insoluble substrates and degrade them to soluble products which are then absorbed. Cellulosome complexes are intricate, multi-enzyme machines, produced by many cellulolytic microorganisms. They are produced by microorganisms for efficient degradation of plant cell wall polysaccharides, notably cellulose, the most abundant organic polymer on Earth. The multiple subunits of cellulosomes are composed of numerous functional domains that interact with each other and with the cellulosic substrate. One of these subunits, a large glycoprotein "scaffoldin", is a distinctive class of non-catalytic scaffolding polypeptides. The scaffoldin subunit selectively integrates the various cellulases and xylanase subunits into the cohesive complex, by combining its cohesin domains with a typical dockerin domain present on each of the subunit enzymes. The scaffoldin of some cellulosomes, an example being that of Clostridium thermocellum, contains a carbohydrate-binding module that adheres cellulose to the cellulosomal complex.

Butanol may be used as a fuel in an internal combustion engine. It is more similar to gasoline than it is to ethanol. A C4-hydrocarbon, butanol is a drop-in fuel and thus works in vehicles designed for use with gasoline without modification. Both n-butanol and isobutanol have been studied as possible fuels. Both can be produced from biomass (as "biobutanol" ) as well as from fossil fuels (as "petrobutanol"). The chemical properties depend on the isomer (n-butanol or isobutanol), not on the production method.
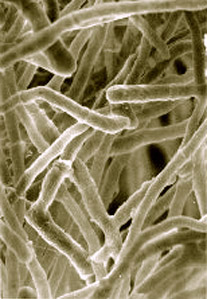
Trichoderma reesei is a mesophilic and filamentous fungus. It is an anamorph of the fungus Hypocrea jecorina. T. reesei can secrete large amounts of cellulolytic enzymes. Microbial cellulases have industrial application in the conversion of cellulose, a major component of plant biomass, into glucose.
Neutralite Avidin protein is a deglycosylated version of chicken avidin, with a mass of approximately 60,000 daltons. As a result of carbohydrate removal, lectin binding is reduced to undetectable levels, yet biotin binding affinity is retained because the carbohydrate is not necessary for this activity. Avidin has a high pI but NeutrAvidin has a near-neutral pI, minimizing non-specific interactions with the negatively-charged cell surface or with DNA/RNA. Neutravidin still has lysine residues that remain available for derivatization or conjugation.

Meir Wilchek is an Israeli biochemist. He is a professor at the Weizmann Institute of Science.
Fibrobacter succinogenes is a cellulolytic bacterium species in the genus Fibrobacter. It is present in the rumen of cattle. F. succinogenes is a gram negative, rod-shaped, obligate anaerobe that is a major contributor to cellulose digestion. Since its discovery in the 1950s, it has been studied for its role in herbivore digestion and cellulose fermentation, which can be utilized in biofuel production.

Dockerin is a protein domain found in the cellulosome cellular structure of anaerobic bacteria. It is found on many endoglucanase enzymes. The dockerin's binding partner is the cohesin domain, located on the scaffoldin protein. This interaction between the dockerin domains of the enzyme constituents of the cellulosome and the cohesin domains of the scaffoldin protein is essential to the construction of the cellulosome complex. The Dockerin domain has two in-tandem repeats of a non-EF hand calcium binding motif. Each motif is characterized by a loop-helix structure. The three-dimensional structure of dockerin has been determined in solution, as well as in complex with Cohesin.

In molecular biology, a carbohydrate-binding module (CBM) is a protein domain found in carbohydrate-active enzymes. The majority of these domains have carbohydrate-binding activity. Some of these domains are found on cellulosomal scaffoldin proteins. CBMs were previously known as cellulose-binding domains. CBMs are classified into numerous families, based on amino acid sequence similarity. There are currently 64 families of CBM in the CAZy database.

In molecular biology, the cohesin domain is a protein domain. It interacts with a complementary domain, termed the dockerin domain. The cohesin-dockerin interaction is the crucial interaction for complex formation in the cellulosome.
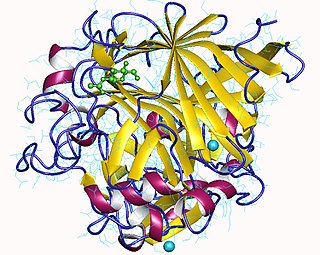
Cellulose 1,4-β-cellobiosidase is an enzyme of interest for its capability of converting cellulose to useful chemicals, particularly cellulosic ethanol.
Acetivibrio clariflavus is an anaerobic bacterium from the genus Acetivibrio which has been isolated from sludge from a cellulose-degrading bioreactor in Japan.
Eric A. Johnson is a microbiologist and an academic. He is a retired Professor of Bacteriology from the University of Wisconsin-Madison, serving from 1985 to 2020.