
Cellulase is any of several enzymes produced chiefly by fungi, bacteria, and protozoans that catalyze cellulolysis, the decomposition of cellulose and of some related polysaccharides. The name is also used for any naturally occurring mixture or complex of various such enzymes, that act serially or synergistically to decompose cellulosic material.

Beta-glucosidase is an enzyme that catalyzes the hydrolysis of the glycosidic bonds to terminal non-reducing residues in beta-D-glucosides and oligosaccharides, with release of glucose.
Cellodextrins are glucose polymers of varying length resulting from cellulolysis, the breakdown of cellulose.
Neocallimastigomycota is a phylum containing anaerobic fungi, which are symbionts found in the digestive tracts of larger herbivores. Anaerobic fungi were originally placed within phylum Chytridiomycota, within Order Neocallimastigales but later raised to phylum level, a decision upheld by later phylogenetic reconstructions. It encompasses only one family.
Candex is a dietary supplement manufactured by Pure Essence Laboratories. It is marketed as an enzymatic remedy to treat the yeast infection candida. Having the status of a dietary supplement, its efficiency has not been proven in scientifically controlled and peer-reviewed trials. Similar formulas exist, such as Candigest.
Clostridium thermocellum is an anaerobic, thermophilic bacterium. C. thermocellum has garnered research interest due to its cellulolytic and ethanologenic abilities, being capable of directly converting a cellulosic substrate into ethanol by consolidated bioprocessing. This makes it useful in converting biomass into a usable energy source. The degradation of the cellulose is carried out in the bacterium by a large extracellular cellulase system called a cellulosome, which contains nearly 20 catalytic subunits. The cellulase system of the bacterium significantly differs from fungal cellulases due to its high activity on crystalline cellulose, being able to completely solubilize crystalline sources of cellulose, such as cotton. However, there are some shortfalls in applying the organism to practical applications due to it having low ethanol yield, at least partially due to branched fermentation pathways that produce acetate, formate, and lactate along with ethanol. There is also evidence of inhibition due to the presence of hydrogen and due to agitation. Some recent research has been directed to optimizing the ethanol-producing metabolic pathway in hopes of creating more efficient biomass conversion.

Dockerin is a protein domain found in the cellulosome cellular structure of anaerobic bacteria. It is found on many endoglucanase enzymes. The dockerin's binding partner is the cohesin domain, located on the scaffoldin protein. This interaction between the dockerin domains of the enzyme constituents of the cellulosome and the cohesin domains of the scaffoldin protein is essential to the construction of the cellulosome complex. The Dockerin domain has two in-tandem repeats of a non-EF hand calcium binding motif. Each motif is characterized by a loop-helix structure. The three-dimensional structure of dockerin has been determined in solution, as well as in complex with Cohesin.
Butyrivibrio is a genus of bacteria in Class Clostridia. Bacteria of this genus are common in the gastrointestinal systems of many animals. Genus Butyrivibrio was first described by Bryant and Small (1956) as anaerobic, butyric acid-producing, curved rods. Butyrivibrio cells are small, typically 0.4 – 0.6 µm by 2 – 5 µm. They are motile, using a single polar or subpolar monotrichous flagellum. They are commonly found singly or in short chains but it is not unusual for them to form long chains. Despite historically being described as Gram-negative, their cell walls contain derivatives of teichoic acid, and electron microscopy indicates that bacteria of this genus have a Gram-positive cell wall type. It is thought that they appear Gram-negative when Gram stained because their cell walls thin to 12 to 18 nm as they reach stationary phase.
Ruminococcus is a genus of bacteria in the class Clostridia. They are anaerobic, Gram-positive gut microbes. One or more species in this genus are found in significant numbers in the human gut microbiota. The type species is R. flavefaciens. As usual, bacteria taxonomy is in flux, with Clostridia being paraphyletic, and some erroneous members of Ruminococcus being reassigned to a new genus Blautia on the basis of 16S rRNA gene sequences.

In molecular biology, a carbohydrate-binding module (CBM) is a protein domain found in carbohydrate-active enzymes. The majority of these domains have carbohydrate-binding activity. Some of these domains are found on cellulosomal scaffoldin proteins. CBMs were previously known as cellulose-binding domains. CBMs are classified into numerous families, based on amino acid sequence similarity. There are currently 64 families of CBM in the CAZy database.
Fibrolytic bacteria constitute a group of microorganisms that are able to process complex plant polysaccharides thanks to their capacity to synthesize cellulolytic and hemicellulolytic enzymes. Polysaccharides are present in plant cellular cell walls in a compact fiber form where they are mainly composed of cellulose and hemicellulose.

In molecular biology, the cohesin domain is a protein domain. It interacts with a complementary domain, termed the dockerin domain. The cohesin-dockerin interaction is the crucial interaction for complex formation in the cellulosome.
Clostridium phytofermentans is an obligately anaerobic, rod-shaped spore-forming, Gram-positive bacterium in the family Lachnospiraceae. It is a model organism of interest for its ability to rapidly ferment diverse plant polysaccharides including cellulose, hemicellulose, and pectin to ethanol, acetate, and hydrogen. The C. phytofermentans 4.8 Mb genome has been fully sequenced, revealing it contains over 170 enzymes in the CAZy database, though one hydrolase appears to be essential for degrading cellulose.
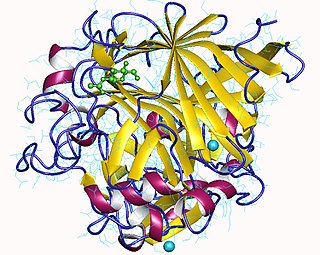
Cellulose 1,4-beta-cellobiosidase is an enzyme of interest for its capability of converting cellulose to useful chemicals, particularly cellulosic ethanol.
Cellulose 1,4-beta-cellobiosidase is an enzyme with systematic name 4-beta-D-glucan cellobiohydrolase . This enzyme catalyses the following chemical reaction
Clostridium cellulovorans is an anaerobic, mesophilic, spore-forming cellulolytic bacterium. Its cells are gram-negative and are non-motile rods which form oblong spores. The type strain is 743B. Its role as an object of study is based on the latter notion.
Thermomyces lanuginosus is a species of thermophilic fungus that belongs to Thermomyces, a genus of hemicellulose degraders. It is classified as a deuteromycete and no sexual form has ever been observed. It is the dominant fungus of compost heaps, due to its ability to withstand high temperatures and use complex carbon sources for energy. As the temperature of compost heaps rises and the availability of simple carbon sources decreases, it is able to out compete pioneer microflora. It plays an important role in breaking down the hemicelluloses found in plant biomass due to the many hydrolytic enzymes that it produces, such as lipolase, amylase, xylanase, phytase, and chitinase. These enzymes have chemical, environmental, and industrial applications due to their hydrolytic properties. They are used in the food, petroleum, pulp and paper, and animal feed industries, among others. A few rare cases of endocarditis due to T. lanuginosus have been reported in humans.
Methanogens are a group of microorganisms that can produce methane as a byproduct of their metabolism.They hold an important place in the digestive system of ruminants. The digestive tract of ruminants contain four major parts, they are abomasum, rumen, omasum and reticulum.The food with saliva is first passed to the rumen for breaking them into smaller particles and then it moves to the reticulum where the food is broken into further smaller particles and the indigestable particles are sent back for rechewing and then to rumen. The majority of the anaerobic microbes assisting the cellulose breakdown occupy the rumen. They initiate the fermentation process.The animal absorbs the fatty acids, vitamins and nutrient content on passing the partially digested food from rumen to omasum which, decreases the pH level and thus initiates the release of enzymes for further break down the food which is later passed to the abomasum that absorbs remaining nutrients before excretion.This process takes about 9–12 hours.

Edward A. Bayer is an American-Israeli scientist.
Clostridium clariflavum is an anaerobic bacterium from the genus of Clostridium which has been isolated from sludge from a cellulose-degrading bioreactor in Japan.






