Related Research Articles

Brine is a high-concentration solution of salt in water. In diverse contexts, brine may refer to the salt solutions ranging from about 3.5% up to about 26%. Brine forms naturally due to evaporation of ground saline water but it is also generated in the mining of sodium chloride. Brine is used for food processing and cooking, for de-icing of roads and other structures, and in a number of technological processes. It is also a by-product of many industrial processes, such as desalination, so it requires wastewater treatment for proper disposal or further utilization.

Desalination is a process that takes away mineral components from saline water. More generally, desalination refers to the removal of salts and minerals from a target substance, as in soil desalination, which is an issue for agriculture. Saltwater is desalinated to produce water suitable for human consumption or irrigation. The by-product of the desalination process is brine. Desalination is used on many seagoing ships and submarines. Most of the modern interest in desalination is focused on cost-effective provision of fresh water for human use. Along with recycled wastewater, it is one of the few rainfall-independent water resources.
Ultrafiltration (UF) is a variety of membrane filtration in which forces such as pressure or concentration gradients lead to a separation through a semipermeable membrane. Suspended solids and solutes of high molecular weight are retained in the so-called retentate, while water and low molecular weight solutes pass through the membrane in the permeate (filtrate). This separation process is used in industry and research for purifying and concentrating macromolecular (103–106 Da) solutions, especially protein solutions.
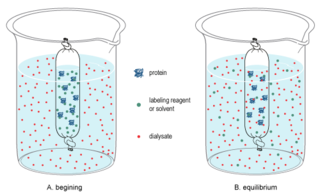
In chemistry, dialysis is the process of separating molecules in solution by the difference in their rates of diffusion through a semipermeable membrane, such as dialysis tubing.

Forward osmosis (FO) is an osmotic process that, like reverse osmosis (RO), uses a semi-permeable membrane to effect separation of water from dissolved solutes. The driving force for this separation is an osmotic pressure gradient, such that a "draw" solution of high concentration, is used to induce a net flow of water through the membrane into the draw solution, thus effectively separating the feed water from its solutes. In contrast, the reverse osmosis process uses hydraulic pressure as the driving force for separation, which serves to counteract the osmotic pressure gradient that would otherwise favor water flux from the permeate to the feed. Hence significantly more energy is required for reverse osmosis compared to forward osmosis.
Solar desalination is a desalination technique powered by solar energy. The two common methods are direct (thermal) and indirect (photovoltaic).

Electrodialysis (ED) is used to transport salt ions from one solution through ion-exchange membranes to another solution under the influence of an applied electric potential difference. This is done in a configuration called an electrodialysis cell. The cell consists of a feed (dilute) compartment and a concentrate (brine) compartment formed by an anion exchange membrane and a cation exchange membrane placed between two electrodes. In almost all practical electrodialysis processes, multiple electrodialysis cells are arranged into a configuration called an electrodialysis stack, with alternating anion and cation-exchange membranes forming the multiple electrodialysis cells. Electrodialysis processes are different from distillation techniques and other membrane based processes in that dissolved species are moved away from the feed stream, whereas other processes move away the water from the remaining substances. Because the quantity of dissolved species in the feed stream is far less than that of the fluid, electrodialysis offers the practical advantage of much higher feed recovery in many applications.

Osmotic power, salinity gradient power or blue energy is the energy available from the difference in the salt concentration between seawater and river water. Two practical methods for this are reverse electrodialysis (RED) and pressure retarded osmosis (PRO). Both processes rely on osmosis with membranes. The key waste product is brackish water. This byproduct is the result of natural forces that are being harnessed: the flow of fresh water into seas that are made up of salt water.
Thin-film composite membranes are semipermeable membranes manufactured to provide selectivity with high permeability. Most TFC's are used in water purification or water desalination systems. They also have use in chemical applications such as gas separations, dehumidification, batteries and fuel cells. A TFC membrane can be considered a molecular sieve constructed in the form of a film from two or more layered materials. The additional layers provide structural strength and a low-defect surface to support a selective layer that is thin enough to be selective but not so thick that it causes low permeability.
Reverse electrodialysis (RED) is the salinity gradient energy retrieved from the difference in the salt concentration between seawater and river water. A method of utilizing the energy produced by this process by means of a heat engine was invented by Prof. Sidney Loeb in 1977 at the Ben-Gurion University of the Negev. --United States Patent US4171409
Reverse osmosis (RO) is a water purification process that uses a semi-permeable membrane to separate water molecules from other substances. RO applies pressure to overcome osmotic pressure that favors even distributions. RO can remove dissolved or suspended chemical species as well as biological substances, and is used in industrial processes and the production of potable water. RO retains the solute on the pressurized side of the membrane and the purified solvent passes to the other side. It relies on the relative sizes of the various molecules to decide what passes through. "Selective" membranes reject large molecules, while accepting smaller molecules.

Pressure retarded osmosis (PRO) is a technique to separate a solvent from a solution that is more concentrated and also pressurized. A semipermeable membrane allows the solvent to pass to the concentrated solution side by osmosis. The technique can be used to generate power from the salinity gradient energy resulting from the difference in the salt concentration between sea and river water. In PRO, the water potential between fresh water and sea water corresponds to a pressure of 26 bars. This pressure is equivalent to a column of water 270 meters high. However, the optimal working pressure is only half of this, 11 to 15 bar.
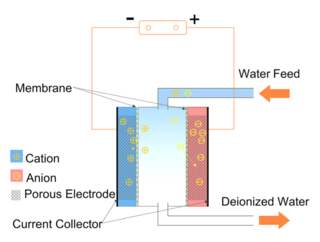
Capacitive deionization (CDI) is a technology to deionize water by applying an electrical potential difference over two electrodes, which are often made of porous carbon. In other words, CDI is an electro-sorption method using a combination of a sorption media and an electrical field to separate ions and charged particles. Anions, ions with a negative charge, are removed from the water and are stored in the positively polarized electrode. Likewise, cations are stored in the cathode, which is the negatively polarized electrode.

A membrane is a selective barrier; it allows some things to pass through but stops others. Such things may be molecules, ions, or other small particles. Membranes can be generally classified into synthetic membranes and biological membranes. Biological membranes include cell membranes ; nuclear membranes, which cover a cell nucleus; and tissue membranes, such as mucosae and serosae. Synthetic membranes are made by humans for use in laboratories and industry.
Electrodeionization (EDI) is a water treatment technology that utilizes DC power, ion exchange membranes, and ion exchange resin to deionize water. EDI is typically employed as a polishing treatment following reverse osmosis (RO). It distinguishes itself from other RO polishing methods, like chemically regenerated mixed beds, by operating continuously without the need for chemical regeneration.

Zero Liquid Discharge(ZLD) is a water treatment process designed to remove liquid waste from a system. The focus of ZLD is to reduce wastewater economically and produce clean water that is suitable for reuse (e.g. irrigation). ZLD systems employ advanced wastewater/desalination treatment technologies to purify and recycle virtually all of the wastewater produced.
Concentration polarization is a term used in the scientific fields of electrochemistry and membrane science.
An ion-exchange membrane is a semi-permeable membrane that transports certain dissolved ions, while blocking other ions or neutral molecules.
Water shortages have become an increasingly pressing concern recently and with recent predictions of a high probability of the current drought turning into a megadrought occurring in the western United States, technologies involving water treatment and processing need to improve. Carbon nanotubes (CNT) have been the subject of extensive studies because they demonstrate a range of unique properties that existing technologies lack. For example, carbon nanotube membranes can demonstrate higher water flux with lower energy than current membranes. These membranes can also filter out particles that are too small for conventional systems which can lead to better water purification techniques and less waste. The largest obstacle facing CNT is processing as it is difficult to produce them in the large quantities that most of these technologies will require.
A microbial desalination cell (MDC) is a biological electrochemical system that implements the use of electro-active bacteria to power desalination of water in situ, resourcing the natural anode and cathode gradient of the electro-active bacteria and thus creating an internal supercapacitor. Available water supply has become a worldwide endemic as only .3% of the earth's water supply is usable for human consumption, while over 99% is sequestered by oceans, glaciers, brackish waters, and biomass. Current applications in electrocoagulation, such as microbial desalination cells, are able to desalinate and sterilize formerly unavailable water to render it suitable for safe water supply. Microbial desalination cells stem from microbial fuel cells, deviating by no longer requiring the use of a mediator and instead relying on the charged components of the internal sludge to power the desalination process. Microbial desalination cells therefore do not require additional bacteria to mediate the catabolism of the substrate during biofilm oxidation on the anodic side of the capacitor. MDCs and other bio-electrical systems are favored over reverse osmosis, nanofiltration and other desalination systems due to lower costs, energy and environmental impacts associated with bio-electrical systems.
References
- 1 2 Katz, William E. (January 1979). "The electrodialysis reversal (EDR) process". Desalination. 28 (1): 31–40. doi:10.1016/S0011-9164(00)88124-2.
- ↑ Panagopoulos, Argyris; Haralambous, Katherine-Joanne; Loizidou, Maria (2019-11-25). "Desalination brine disposal methods and treatment technologies - A review". Science of the Total Environment. 693: 133545. Bibcode:2019ScTEn.693m3545P. doi:10.1016/j.scitotenv.2019.07.351. ISSN 0048-9697. PMID 31374511. S2CID 199387639.
- ↑ Benvenuti, Tatiane; Giacobbo, Alexandre; Scarazzato, Tatiana; de Moraes da Trindade, Carolina; Santana Barros, Kayo (2022). Chapter 14 - Electrodialysis, electrodialysis reversal and capacitive deionization technologies, Advancement in Polymer-Based Membranes for Water Remediation. Elsevier. pp. 505–539. doi:10.1016/B978-0-323-88514-0.00014-0. ISBN 9780323885140.
- ↑ Tanaka, Yoshinobu (2007). Chapter 2 Electrodialysis Reversal, Membrane Science and Technology. Elsevier. pp. 383–404. ISBN 9780444519825.