
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the baryonic mass of the universe.
A density matrix is a matrix that describes the statistical state of a system in quantum mechanics. The probability for any outcome of any well-defined measurement upon a system can be calculated from the density matrix for that system. The extreme points in the set of density matrices are the pure states, which can also be written as state vectors or wavefunctions. Density matrices that are not pure states are mixed states. Any mixed state can be represented as a convex combination of pure states, and so density matrices are helpful for dealing with statistical ensembles of different possible preparations of a quantum system, or situations where a precise preparation is not known, as in quantum statistical mechanics.
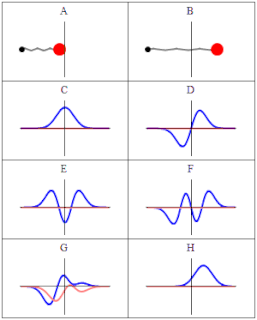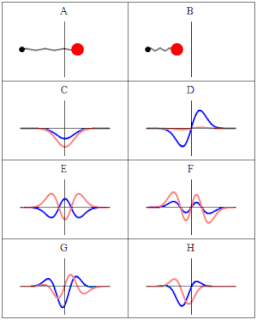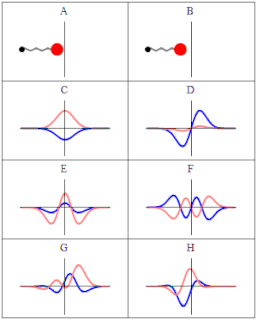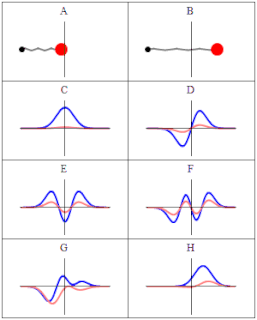
A wave function in quantum physics is a mathematical description of the quantum state of an isolated quantum system. The wave function is a complex-valued probability amplitude, and the probabilities for the possible results of measurements made on the system can be derived from it. The most common symbols for a wave function are the Greek letters ψ and Ψ.
Density-functional theory (DFT) is a computational quantum mechanical modelling method used in physics, chemistry and materials science to investigate the electronic structure of many-body systems, in particular atoms, molecules, and the condensed phases. Using this theory, the properties of a many-electron system can be determined by using functionals, i.e. functions of another function. In the case of DFT, these are functionals of the spatially dependent electron density. DFT is among the most popular and versatile methods available in condensed-matter physics, computational physics, and computational chemistry.

In quantum mechanics, a probability amplitude is a complex number used in describing the behaviour of systems. The modulus squared of this quantity represents a probability or probability density.

The Stark effect is the shifting and splitting of spectral lines of atoms and molecules due to the presence of an external electric field. It is the electric-field analogue of the Zeeman effect, where a spectral line is split into several components due to the presence of the magnetic field. Although initially coined for the static case, it is also used in the wider context to describe the effect of time-dependent electric fields. In particular, the Stark effect is responsible for the pressure broadening of spectral lines by charged particles in plasmas. For most spectral lines, the Stark effect is either linear or quadratic with a high accuracy.
In quantum physics, Fermi's golden rule is a formula that describes the transition rate from one energy eigenstate of a quantum system to a group of energy eigenstates in a continuum, as a result of a weak perturbation. This transition rate is effectively independent of time and is proportional to the strength of the coupling between the initial and final states of the system as well as the density of states. It is also applicable when the final state is discrete, i.e. it is not part of a continuum, if there is some decoherence in the process, like relaxation or collision of the atoms, or like noise in the perturbation, in which case the density of states is replaced by the reciprocal of the decoherence bandwidth.
In physics, a free particle is a particle that, in some sense, is not bound by an external force, or equivalently not in a region where its potential energy varies. In classical physics, this means the particle is present in a "field-free" space. In quantum mechanics, it means a region of uniform potential, usually set to zero in the region of interest since potential can be arbitrarily set to zero at any point in space.
In quantum mechanics, the Hellmann–Feynman theorem relates the derivative of the total energy with respect to a parameter, to the expectation value of the derivative of the Hamiltonian with respect to that same parameter. According to the theorem, once the spatial distribution of the electrons has been determined by solving the Schrödinger equation, all the forces in the system can be calculated using classical electrostatics.
Time-dependent density-functional theory (TDDFT) is a quantum mechanical theory used in physics and chemistry to investigate the properties and dynamics of many-body systems in the presence of time-dependent potentials, such as electric or magnetic fields. The effect of such fields on molecules and solids can be studied with TDDFT to extract features like excitation energies, frequency-dependent response properties, and photoabsorption spectra.

In electromagnetism, charge density is the amount of electric charge per unit length, surface area, or volume. Volume charge density is the quantity of charge per unit volume, measured in the SI system in coulombs per cubic meter (C⋅m−3), at any point in a volume. Surface charge density (σ) is the quantity of charge per unit area, measured in coulombs per square meter (C⋅m−2), at any point on a surface charge distribution on a two dimensional surface. Linear charge density (λ) is the quantity of charge per unit length, measured in coulombs per meter (C⋅m−1), at any point on a line charge distribution. Charge density can be either positive or negative, since electric charge can be either positive or negative.
In quantum mechanics, the probability current is a mathematical quantity describing the flow of probability in terms of probability per unit time per unit area. Specifically, if one describes the probability density as a heterogeneous fluid, then the probability current is the rate of flow of this fluid. This is analogous to mass currents in hydrodynamics and electric currents in electromagnetism. It is a real vector, like electric current density. The concept of a probability current is a useful formalism in quantum mechanics. The probability current is invariant under Gauge Transformation.
In many-body theory, the term Green's function is sometimes used interchangeably with correlation function, but refers specifically to correlators of field operators or creation and annihilation operators.
A hydrogen-like atom/ion (usually called a "hydrogenic atom") is any atomic nucleus bound to one electron and thus is isoelectronic with hydrogen. These atoms or ions can carry the positive charge , where is the atomic number of the atom. Examples of hydrogen-like atoms/ions are hydrogen itself, He+, Li2+, Be3+ and B4+. Because hydrogen-like atoms/ions are two-particle systems with an interaction depending only on the distance between the two particles, their (non-relativistic) Schrödinger equation can be solved in analytic form, as can the (relativistic) Dirac equation. The solutions are one-electron functions and are referred to as hydrogen-like atomic orbitals.
In 1927, a year after the publication of the Schrödinger equation, Hartree formulated what are now known as the Hartree equations for atoms, using the concept of self-consistency that Lindsay had introduced in his study of many electron systems in the context of Bohr theory. Hartree assumed that the nucleus together with the electrons formed a spherically symmetric field. The charge distribution of each electron was the solution of the Schrödinger equation for an electron in a potential , derived from the field. Self-consistency required that the final field, computed from the solutions was self-consistent with the initial field and he called his method the self-consistent field method.
In quantum mechanics, specifically time-dependent density functional theory, the Runge–Gross theorem shows that for a many-body system evolving from a given initial wavefunction, there exists a one-to-one mapping between the potential in which the system evolves and the density of the system. The potentials under which the theorem holds are defined up to an additive purely time-dependent function: such functions only change the phase of the wavefunction and leave the density invariant. Most often the RG theorem is applied to molecular systems where the electronic density, ρ(r,t) changes in response to an external scalar potential, v(r,t), such as a time-varying electric field.
The fractional Schrödinger equation is a fundamental equation of fractional quantum mechanics. It was discovered by Nick Laskin (1999) as a result of extending the Feynman path integral, from the Brownian-like to Lévy-like quantum mechanical paths. The term fractional Schrödinger equation was coined by Nick Laskin.
In quantum physics, a quantum state is a mathematical entity that provides a probability distribution for the outcomes of each possible measurement on a system. Knowledge of the quantum state together with the rules for the system's evolution in time exhausts all that can be predicted about the system's behavior. A mixture of quantum states is again a quantum state. Quantum states that cannot be written as a mixture of other states are called pure quantum states, while all other states are called mixed quantum states. A pure quantum state can be represented by a ray in a Hilbert space over the complex numbers, while mixed states are represented by density matrices, which are positive semidefinite operators that act on Hilbert spaces.
In quantum mechanics, the variational method is one way of finding approximations to the lowest energy eigenstate or ground state, and some excited states. This allows calculating approximate wavefunctions such as molecular orbitals. The basis for this method is the variational principle.

Angular Correlation of Electron Positron Annihilation Radiation is a technique of solid state physics to investigate the electronic structure of metals. It uses positrons which are implanted into a sample and annihilate with the electrons. In the majority of annihilation events, two gamma quanta are created that are, in the reference frame of the electron-positron pair, emitted in exactly opposite directions. In the laboratory frame, there is a small angular deviation from collinearity, which is caused by the momentum of the electron. Hence, measuring the angular correlation of the annihilation radiation yields information about the momentum distribution of the electrons in the solid.































