Related Research Articles

Microscopy is the technical field of using microscopes to view objects and areas of objects that cannot be seen with the naked eye. There are three well-known branches of microscopy: optical, electron, and scanning probe microscopy, along with the emerging field of X-ray microscopy.

Optical coherence tomography (OCT) is an imaging technique that uses interferometry with short-coherence-length light to obtain micrometer-level depth resolution and uses transverse scanning of the light beam to form two- and three-dimensional images from light reflected from within biological tissue or other scattering media. Short-coherence-length light can be obtained using a superluminescent diode (SLD) with a broad spectral bandwidth or a broadly tunable laser with narrow linewidth. The first demonstration of OCT imaging was published by a team from MIT and Harvard Medical School in a 1991 article in the journal Science. The article introduced the term "OCT" to credit its derivation from optical coherence-domain reflectometry, in which the axial resolution is based on temporal coherence. The first demonstrations of in vivo OCT imaging quickly followed.

Confocal microscopy, most frequently confocal laser scanning microscopy (CLSM) or laser scanning confocal microscopy (LSCM), is an optical imaging technique for increasing optical resolution and contrast of a micrograph by means of using a spatial pinhole to block out-of-focus light in image formation. Capturing multiple two-dimensional images at different depths in a sample enables the reconstruction of three-dimensional structures within an object. This technique is used extensively in the scientific and industrial communities and typical applications are in life sciences, semiconductor inspection and materials science.

Two-photon excitation microscopy is a fluorescence imaging technique that is particularly well-suited to image scattering living tissue of up to about one millimeter in thickness. Unlike traditional fluorescence microscopy, where the excitation wavelength is shorter than the emission wavelength, two-photon excitation requires simultaneous excitation by two photons with longer wavelength than the emitted light. The laser is focused onto a specific location in the tissue and scanned across the sample to sequentially produce the image. Due to the non-linearity of two-photon excitation, mainly fluorophores in the micrometer-sized focus of the laser beam are excited, which results in the spatial resolution of the image. This contrasts with confocal microscopy, where the spatial resolution is produced by the interaction of excitation focus and the confined detection with a pinhole.

Photoacoustic imaging or optoacoustic imaging is a biomedical imaging modality based on the photoacoustic effect. Non-ionizing laser pulses are delivered into biological tissues and part of the energy will be absorbed and converted into heat, leading to transient thermoelastic expansion and thus wideband ultrasonic emission. The generated ultrasonic waves are detected by ultrasonic transducers and then analyzed to produce images. It is known that optical absorption is closely associated with physiological properties, such as hemoglobin concentration and oxygen saturation. As a result, the magnitude of the ultrasonic emission, which is proportional to the local energy deposition, reveals physiologically specific optical absorption contrast. 2D or 3D images of the targeted areas can then be formed.

Bruce J. Tromberg is an American photochemist and a leading researcher in the field of biophotonics. He is the director of the National Institute of Biomedical Imaging and Bioengineering (NIBIB) within the National Institutes of Health (NIH). Before joining NIH, he was Professor of Biomedical Engineering at The Henry Samueli School of Engineering and of Surgery at the School of Medicine, University of California, Irvine. He was the principal investigator of the Laser Microbeam and Medical Program (LAMMP), and the Director of the Beckman Laser Institute and Medical Clinic at Irvine. He was a co-leader of the Onco-imaging and Biotechnology Program of the NCI Chao Family Comprehensive Cancer Center at Irvine.

Second-harmonic imaging microscopy (SHIM) is based on a nonlinear optical effect known as second-harmonic generation (SHG). SHIM has been established as a viable microscope imaging contrast mechanism for visualization of cell and tissue structure and function. A second-harmonic microscope obtains contrasts from variations in a specimen's ability to generate second-harmonic light from the incident light while a conventional optical microscope obtains its contrast by detecting variations in optical density, path length, or refractive index of the specimen. SHG requires intense laser light passing through a material with a noncentrosymmetric molecular structure, either inherent or induced externally, for example by an electric field.

The Raman microscope is a laser-based microscopic device used to perform Raman spectroscopy. The term MOLE is used to refer to the Raman-based microprobe. The technique used is named after C. V. Raman, who discovered the scattering properties in liquids.
Endomicroscopy is a technique for obtaining histology-like images from inside the human body in real-time, a process known as ‘optical biopsy’. It generally refers to fluorescence confocal microscopy, although multi-photon microscopy and optical coherence tomography have also been adapted for endoscopic use. Commercially available clinical and pre-clinical endomicroscopes can achieve a resolution on the order of a micrometre, have a field-of-view of several hundred μm, and are compatible with fluorophores which are excitable using 488 nm laser light. The main clinical applications are currently in imaging of the tumour margins of the brain and gastro-intestinal tract, particularly for the diagnosis and characterisation of Barrett’s Esophagus, pancreatic cysts and colorectal lesions. A number of pre-clinical and transnational applications have been developed for endomicroscopy as it enables researchers to perform live animal imaging. Major pre-clinical applications are in gastro-intestinal tract, toumour margin detection, uterine complications, ischaemia, live imaging of cartilage and tendon and organoid imaging.
The Beckman Laser Institute is an interdisciplinary research center for the development of optical technologies and their use in biology and medicine. Located on the campus of the University of California, Irvine in Irvine, California, an independent nonprofit corporation was created in 1982, under the leadership of Michael W. Berns, and the actual facility opened on June 4, 1986. It is one of a number of institutions focused on translational research, connecting research and medical applications. Researchers at the institute have developed laser techniques for the manipulation of structures within a living cell, and applied them medically in treatment of skin conditions, stroke, and cancer, among others.
Lihong V. Wang is the Bren Professor of Medical Engineering and Electrical Engineering at the Andrew and Peggy Cherng Department of Medical Engineering at California Institute of Technology and was formerly the Gene K. Beare Distinguished Professorship of Biomedical Engineering at Washington University in St. Louis. Wang is known for his contributions to the field of Photoacoustic imaging technologies. Wang was elected as the member of National Academy of Engineering (NAE) in 2018.

Endoscopic optical coherence tomography, also intravascular optical coherence tomography is a catheter-based imaging application of optical coherence tomography (OCT). It is capable of acquiring high-resolution images from inside a blood vessel using optical fibers and laser technology.
Three-photon microscopy (3PEF) is a high-resolution fluorescence microscopy based on nonlinear excitation effect. Different from two-photon excitation microscopy, it uses three exciting photons. It typically uses 1300 nm or longer wavelength lasers to excite the fluorescent dyes with three simultaneously absorbed photons. The fluorescent dyes then emit one photon whose energy is three times the energy of each incident photon. Compared to two-photon microscopy, three-photon microscopy reduces the fluorescence away from the focal plane by , which is much faster than that of two-photon microscopy by . In addition, three-photon microscopy employs near-infrared light with less tissue scattering effect. This causes three-photon microscopy to have higher resolution than conventional microscopy.

Gail McConnell is a Scottish physicist who is Professor of Physics and director of the Centre for Biophotonics at the University of Strathclyde. She is interested in optical microscopy and novel imaging techniques, and leads the Mesolens microscope facility where her research investigates linear and non-linear optics.

Anita Mahadevan-Jansen is a Professor of Biomedical Engineering and holds the Orrin H. Ingram Chair in Biomedical Engineering at Vanderbilt University. Her research considers the development of optical techniques for clinical diagnosis and surgical guidance, particularly using Raman and fluorescence spectroscopy. She serves on the Board of Directors of SPIE, and is a Fellow of SPIE, The Optical Society, Society for Applied Spectroscopy, and the American Society for Lasers in Medicine and Surgery. She was elected to serve as the 2020 Vice President of SPIE. With her election, Mahadevan-Jansen joined the SPIE presidential chain and served as President-Elect in 2021 and the Society's President in 2022.
Kristen Carlson Maitland is an associate professor at Texas A&M University. She develops optical instrumentation for the detection and diagnosis of diseases, including infection and cancer. She has served on the Board of Directors of SPIE.
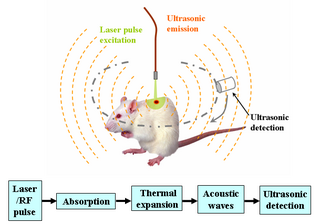
Deep learning in photoacoustic imaging combines the hybrid imaging modality of photoacoustic imaging (PA) with the rapidly evolving field of deep learning. Photoacoustic imaging is based on the photoacoustic effect, in which optical absorption causes a rise in temperature, which causes a subsequent rise in pressure via thermo-elastic expansion. This pressure rise propagates through the tissue and is sensed via ultrasonic transducers. Due to the proportionality between the optical absorption, the rise in temperature, and the rise in pressure, the ultrasound pressure wave signal can be used to quantify the original optical energy deposition within the tissue.

Igor Meglinski is a British, New Zealand and Finnish scientist serving as a principal investigator at the College of Engineering & Physical Sciences at Aston University, where he is a Professor in Quantum Biophotonics and Biomedical Engineering. He is a Faculty member in the School of Engineering and Technology at the Department of Mechanical, Biomedical & Design Engineering, and is also associated with the Aston Institute of Photonic Technologies (AIPT) and Aston Research Centre for Health in Ageing (ARCHA).
Nozomi Nishimura is an American biomedical engineer who is an associate professor at Cornell University. She was awarded the L'Oréal for Women in Science Fellowship in 2009 and was inducted into the 2024 Class of the AIMBE College of Fellows for her research in intravital microscopy contributing to the understanding of microscale physiology.

Lingyan Shi is an associate professor in the Shu Chien-Gene Lay Department of Bioengineering in Jacobs School of Engineering, University of California, San Diego.
References
- 1 2 3 Hillman, Elizabeth (2002). Experimental and theoretical investigations of near infrared tomographic imaging methods and clinical applications (PDF) (PhD thesis). University College London. OCLC 1000838839. EThOS uk.bl.ethos.268884. Archived from the original (PDF) on 2014-05-08. Retrieved 2018-08-19.
- ↑ Elizabeth Hillman publications indexed by Google Scholar
- 1 2 3 4 5 "Hillman Lab: Biomedical Engineering: Columbia University: Elizabeth Hillman". orion.bme.columbia.edu. Archived from the original on 2018-08-29. Retrieved 2018-08-20.
- ↑ "Elizabeth Hillman". datascience.columbia.edu. Retrieved 2018-08-20.
- ↑ Hebden, Jeremy; Bland, T.; Hillman, Elizabeth M. C.; Gibson, A.; Everdell, N.; Delpy, David T.; Arridge, Simon R.; Douek, M. (2002-04-07). "Optical tomography of the breast using a 32-channel time-resolved imager". Biomedical Topical Meeting. pp. SuE5. doi:10.1364/BIO.2002.SuE5. ISBN 1-55752-702-4.
- ↑ Hebden, Jeremy C.; Schmidt, Florian E. W.; Fry, Martin E.; Hillman, Elizabeth M. C.; Schweiger, Martin; Delpy, David T. (1999-06-14). "Imaging of tissue-equivalent phantoms using the UCL multi-channel time-resolved instrument". Biomedical Optics. pp. AMC4. doi:10.1364/BIO.1999.AMC4.
- ↑ Hillman, Elizabeth M. C.; Moore, Anna (2007-08-19). "All-optical anatomical co-registration for molecular imaging of small animals using dynamic contrast". Nature Photonics. 1 (9): 526–530. Bibcode:2007NaPho...1..526H. doi:10.1038/nphoton.2007.146. ISSN 1749-4885. PMC 2575379 . PMID 18974848.
- ↑ "Professor Elizabeth Hillman wins OSA Adolph Lomb Medal". bme.columbia.edu. Archived from the original on 2018-06-21. Retrieved 2018-08-20.
- ↑ "NSF Award Search: Award#0954796 - CAREER: Interventional Microscopy for In-vivo Investigations of Brain Function". www.nsf.gov. Retrieved 2018-08-20.
- ↑ Hillman, Elizabeth M. C.; Amoozegar, Cyrus B.; Wang, Tracy; McCaslin, Addason F. H.; Bouchard, Matthew B.; Mansfield, James; Levenson, Richard M. (2011-11-28). "In vivo optical imaging and dynamic contrast methods for biomedical research". Phil. Trans. R. Soc. A. 369 (1955): 4620–4643. Bibcode:2011RSPTA.369.4620H. doi:10.1098/rsta.2011.0264. ISSN 1364-503X. PMC 3263788 . PMID 22006910.
- ↑ "Making light work: illuminating the future of biomedical optics | Royal Society". royalsociety.org. Retrieved 2018-08-20.
- ↑ "Grantome: Search". Grantome. Retrieved 2018-08-20.
- ↑ "ÜberResearch - Optics and the Brain Topical Meeting". grants.uberresearch.com. Retrieved 2018-08-20.
- ↑ "Brain power: New insight into how the brain regulates its blood flow" . Retrieved 2018-08-20.
- ↑ Hillman, Elizabeth M. C. (2014-06-12). "Out for Blood". Scientific American Mind. 25 (4): 58–65. doi:10.1038/scientificamericanmind0714-58. ISSN 1555-2284.
- ↑ "Elizabeth M.C. Hillman to be Inducted into Medical and Biological Engineering Elite" (PDF). AIMBE. Retrieved 2018-08-20.
- ↑ Center, Laser Biomedical Research (2018-03-19), 2/27/18: Elizabeth M.C. Hillman - "High-speed imaging of whole-brain activity" (3/3) , retrieved 2018-08-20
- ↑ "ÜberResearch - SCAPE microscopy for high-speed in-vivo volumetric microscopy in behaving organisms". grants.uberresearch.com. Retrieved 2018-08-20.
- ↑ Yu, Hang; Galwaduge, P. Thilanka; Voleti, Venkatakaushik; Patel, Kripa B.; Shaik, Mohammed A.; Li, Wenze; Hillman, Elizabeth M. C. (2018-04-03). "Combining Near-infrared Excitation with Swept Confocally-aligned Planar Excitation (SCAPE) Microscopy for Fast, Volumetric Imaging in Mouse Brain". Biophotonics Congress: Biomedical Optics Congress 2018 (Microscopy/Translational/Brain/OTS). pp. BF3C.3. doi:10.1364/BRAIN.2018.BF3C.3. ISBN 978-1-943580-41-5.
- ↑ Bouchard, Matthew B.; Voleti, Venkatakaushik; Mendes, César S.; Lacefield, Clay; Grueber, Wesley B.; Mann, Richard S.; Bruno, Randy M.; Hillman, Elizabeth M. C. (2015-01-19). "Swept confocally-aligned planar excitation (SCAPE) microscopy for high-speed volumetric imaging of behaving organisms". Nature Photonics. 9 (2): 113–119. Bibcode:2015NaPho...9..113B. doi:10.1038/nphoton.2014.323. ISSN 1749-4885. PMC 4317333 . PMID 25663846.
- ↑ "Hillman Lab: Biomedical Engineering: Columbia University: Research". orion.bme.columbia.edu. Archived from the original on 2018-09-01. Retrieved 2018-08-20.
- ↑ "Women in Optics | Video Interviews". SPIE . Retrieved 2018-08-20.
- ↑ Hillman, Elizabeth M. C.; Burgess, Sean A. (2009-02-01). "Sub-millimeter resolution 3D optical imaging of living tissue using laminar optical tomography". Laser & Photonics Reviews. 3 (1–2): 159–179. Bibcode:2009LPRv....3..159H. doi:10.1002/lpor.200810031. ISSN 1863-8880. PMC 2763333 . PMID 19844595.
- 1 2 3 4 5 6 "Hillman Lab: Biomedical Engineering: Columbia University: Elizabeth Hillman". orion.bme.columbia.edu. Archived from the original on 2018-08-29. Retrieved 2020-05-10.
- ↑ "Elizabeth M.C. Hillman | Optica". www.optica.org. Retrieved 2024-08-27.