Related Research Articles

Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large proportion of all proteins are part of this category. For instance, at least 1000 human proteins contain zinc-binding protein domains although there may be up to 3000 human zinc metalloproteins.
Nitrogenases are enzymes (EC 1.18.6.1EC 1.19.6.1) that are produced by certain bacteria, such as cyanobacteria (blue-green bacteria). These enzymes are responsible for the reduction of nitrogen (N2) to ammonia (NH3). Nitrogenases are the only family of enzymes known to catalyze this reaction, which is a key step in the process of nitrogen fixation. Nitrogen fixation is required for all forms of life, with nitrogen being essential for the biosynthesis of molecules (nucleotides, amino acids) that create plants, animals and other organisms. They are encoded by the Nif genes or homologs. They are related to protochlorophyllide reductase.
Bioinorganic chemistry is a field that examines the role of metals in biology. Bioinorganic chemistry includes the study of both natural phenomena such as the behavior of metalloproteins as well as artificially introduced metals, including those that are non-essential, in medicine and toxicology. Many biological processes such as respiration depend upon molecules that fall within the realm of inorganic chemistry. The discipline also includes the study of inorganic models or mimics that imitate the behaviour of metalloproteins.
Artificial photosynthesis is a chemical process that biomimics the natural process of photosynthesis to convert sunlight, water, and carbon dioxide into carbohydrates and oxygen. The term artificial photosynthesis is commonly used to refer to any scheme for capturing and storing the energy from sunlight in the chemical bonds of a fuel. Photocatalytic water splitting converts water into hydrogen and oxygen and is a major research topic of artificial photosynthesis. Light-driven carbon dioxide reduction is another process studied that replicates natural carbon fixation.
Iron–sulfur proteins are proteins characterized by the presence of iron–sulfur clusters containing sulfide-linked di-, tri-, and tetrairon centers in variable oxidation states. Iron–sulfur clusters are found in a variety of metalloproteins, such as the ferredoxins, as well as NADH dehydrogenase, hydrogenases, coenzyme Q – cytochrome c reductase, succinate – coenzyme Q reductase and nitrogenase. Iron–sulfur clusters are best known for their role in the oxidation-reduction reactions of electron transport in mitochondria and chloroplasts. Both Complex I and Complex II of oxidative phosphorylation have multiple Fe–S clusters. They have many other functions including catalysis as illustrated by aconitase, generation of radicals as illustrated by SAM-dependent enzymes, and as sulfur donors in the biosynthesis of lipoic acid and biotin. Additionally, some Fe–S proteins regulate gene expression. Fe–S proteins are vulnerable to attack by biogenic nitric oxide, forming dinitrosyl iron complexes. In most Fe–S proteins, the terminal ligands on Fe are thiolate, but exceptions exist.
A hydrogenase is an enzyme that catalyses the reversible oxidation of molecular hydrogen (H2), as shown below:

Methane monooxygenase (MMO) is an enzyme capable of oxidizing the C-H bond in methane as well as other alkanes. Methane monooxygenase belongs to the class of oxidoreductase enzymes.

Ronald Charles D. Breslow was an American chemist from Rahway, New Jersey. He was University Professor at Columbia University, where he was based in the Department of Chemistry and affiliated with the Departments of Biological Sciences and Pharmacology; he had also been on the faculty of its Department of Chemical Engineering. He had taught at Columbia since 1956 and was a former chair of the university's chemistry department.
The National Academy of Sciences Award in Chemical Sciences is awarded for innovative research in the chemical sciences that in the broadest sense contributes to a better understanding of the natural sciences and to the benefit of humanity.
Bioorganometallic chemistry is the study of biologically active molecules that contain carbon directly bonded to metals or metalloids. The importance of main-group and transition-metal centers has long been recognized as important to the function of enzymes and other biomolecules. However, only a small subset of naturally-occurring metal complexes and synthetically prepared pharmaceuticals are organometallic; that is, they feature a direct covalent bond between the metal(loid) and a carbon atom. The first, and for a long time, the only examples of naturally occurring bioorganometallic compounds were the cobalamin cofactors (vitamin B12) in its various forms. Due to the recent (21st century) discovery of new systems containing carbon-metal bonds in biology, bioorganometallic chemistry is rapidly emerging as a distinct subdiscipline of bioinorganic chemistry that straddles organometallic chemistry and biochemistry. Naturally occurring bioorganometallics include enzymes and sensor proteins. Also within this realm are synthetically prepared organometallic compounds that serve as new drugs and imaging agents (technetium-99m sestamibi) as well as the principles relevant to the toxicology of organometallic compounds (e.g., methylmercury). Consequently, bioorganometallic chemistry is increasingly relevant to medicine and pharmacology.
A hydrogenase mimic or bio-mimetic is an enzyme mimic of hydrogenases.
Biomimetic materials are materials developed using inspiration from nature. This may be useful in the design of composite materials. Natural structures have inspired and innovated human creations. Notable examples of these natural structures include: honeycomb structure of the beehive, strength of spider silks, bird flight mechanics, and shark skin water repellency. The etymological roots of the neologism biomimetic derive from Greek, since bios means "life" and mimetikos means "imitative",
Richard Hadley Holm, also known as R. H. Holm, is an American inorganic chemist.

Stephen James Lippard is the Arthur Amos Noyes Emeritus Professor of Chemistry at the Massachusetts Institute of Technology. He is considered one of the founders of bioinorganic chemistry, studying the interactions of nonliving substances such as metals with biological systems. He is also considered a founder of metalloneurochemistry, the study of metal ions and their effects in the brain and nervous system. He has done pioneering work in understanding protein structure and synthesis, the enzymatic functions of methane monooxygenase (MMO), and the mechanisms of cisplatin anticancer drugs. His work has applications for the treatment of cancer, for bioremediation of the environment, and for the development of synthetic methanol-based fuels.
Amy C. Rosenzweig is a professor of Chemistry and Molecular Biosciences at Northwestern University. She was born in 1967 in Pittsburgh, Pennsylvania. She received her BA in chemistry from Amherst College in 1988, and her Ph.D. from Massachusetts Institute of Technology in 1994. At MIT, Rosenzweig worked under the supervision of Stephen J. Lippard where she pioneered the structural studies of the hydroxylase component of methane monooxygenase from methyloccous capsulatus. Her current research interests include structural biology and bioinorganic chemistry, metal uptake and transport, oxygen activation by metalloenzymes, and characterization of membrane protein. For her work, she has been recognized by a number of national and international awards, including the MacArthur "Genius" Award in 2003.
Marcetta York Darensbourg is an American inorganic chemist. She is a Distinguished Professor of Chemistry at Texas A&M University. Her current work focuses on iron hydrogenases and iron nitrosyl complexes.
[NiFe] hydrogenase is a type of hydrogenase, which is an oxidative enzyme that reversibly convert molecular hydrogen in prokaryotes including Bacteria and Archaea. The catalytic site on the enzyme provides simple hydrogen-metabolizing microorganisms a redox mechanism by which to store and utilize energy via the reaction shown in Figure 1. This is particularly essential for the anaerobic, sulfate-reducing bacteria of the genus Desulfovibrio as well as pathogenic organisms Escherichia coli and Helicobacter pylori. The mechanisms, maturation, and function of [NiFe] hydrogenases are actively being researched for applications to the hydrogen economy and as potential antibiotic targets.
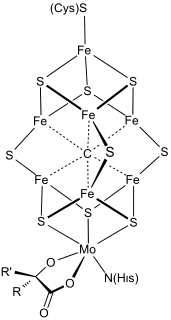
FeMoco (FeMo cofactor) is the primary cofactor of nitrogenase. Nitrogenase is the enzyme that catalyzes the conversion of atmospheric nitrogen molecules N2 into ammonia (NH3) through the process known as nitrogen fixation. Containing iron and molybdenum, the cofactor is called FeMoco. Its stoichiometry is Fe7MoS9C.

Wolfgang Lubitz is a German chemist and biophysicist. He is currently a director emeritus at the Max Planck Institute for Chemical Energy Conversion. He is well known for his work on bacterial photosynthetic reaction centres, hydrogenase enzymes, and the oxygen-evolving complex using a variety of biophysical techniques. He has been recognized by a Festschrift for his contributions to electron paramagnetic resonance (EPR) and its applications to chemical and biological systems.
Serena DeBeer is an American chemist. She is currently a W3-Professor and the director at the Max Planck Institute for Chemical Energy Conversion in Muelheim an der Ruhr, Germany, where she heads the Department of Inorganic Spectroscopy. Her expertise lies in the application and development of X-ray based spectroscopic methods as probes of electronic structure in biological and chemical catalysis.
References
- ↑ "Breslow Group Homepage". www.columbia.edu. Retrieved 2015-12-11.
- ↑ Breslow, Ronald (1995-03-01). "Biomimetic Chemistry and Artificial Enzymes: Catalysis by Design". Accounts of Chemical Research. 28 (3): 146–153. doi:10.1021/ar00051a008. ISSN 0001-4842.
- 1 2 Breslow, Ronald; Overman, Larry E. (1970-02-01). ""Artificial enzyme" combining a metal catalytic group and a hydrophobic binding cavity". Journal of the American Chemical Society. 92 (4): 1075–1077. doi:10.1021/ja00707a062. ISSN 0002-7863.
- ↑ "Wiley: Artificial Enzymes - Ronald Breslow". as.wiley.com. Retrieved 2015-12-11.
- ↑ Kirby, Anthony J; Hollfelder, Florian. "From Enzyme Models to Model Enzymes (RSC Publishing) Anthony J Kirby, Florian Hollfelder". pubs.rsc.org. Retrieved 2015-12-11.
- ↑ Stephen J. Lippard, Jeremy M. Berg, Principles of Bioinorganic Chemistry, University Science Books, 1994, ISBN 0-935702-72-5