
A liposome is a small artificial vesicle, spherical in shape, having at least one lipid bilayer. Due to their hydrophobicity and/or hydrophilicity, biocompatibility, particle size and many other properties, liposomes can be used as drug delivery vehicles for administration of pharmaceutical drugs and nutrients, such as lipid nanoparticles in mRNA vaccines, and DNA vaccines. Liposomes can be prepared by disrupting biological membranes.

A transdermal patch is a medicated adhesive patch that is placed on the skin to deliver a specific dose of medication through the skin and into the bloodstream. An advantage of a transdermal drug delivery route over other types of medication delivery is that the patch provides a controlled release of the medication into the patient, usually through either a porous membrane covering a reservoir of medication or through body heat melting thin layers of medication embedded in the adhesive. The main disadvantage to transdermal delivery systems stems from the fact that the skin is a very effective barrier; as a result, only medications whose molecules are small enough to penetrate the skin can be delivered by this method. The first commercially available prescription patch was approved by the U.S. Food and Drug Administration in December 1979. These patches administered scopolamine for motion sickness.
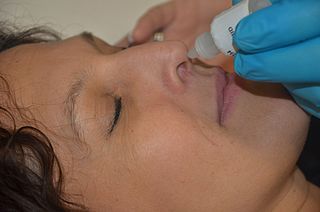
A topical medication is a medication that is applied to a particular place on or in the body. Most often topical medication means application to body surfaces such as the skin or mucous membranes to treat ailments via a large range of classes including creams, foams, gels, lotions, and ointments. Many topical medications are epicutaneous, meaning that they are applied directly to the skin. Topical medications may also be inhalational, such as asthma medications, or applied to the surface of tissues other than the skin, such as eye drops applied to the conjunctiva, or ear drops placed in the ear, or medications applied to the surface of a tooth. The word topical derives from Greek τοπικόςtopikos, "of a place".

1-Decanol is a straight chain fatty alcohol with ten carbon atoms and the molecular formula C10H21OH. It is a colorless to light yellow viscous liquid that is insoluble in water and has an aromatic odor. The interfacial tension against water at 20 °C is 8.97 mN/m.
Dz13 is an experimental treatment developed by scientists at the University of New South Wales. The drug aims to combat a range of illnesses, including skin cancer, restenosis, arthritis and macular degeneration. Trials of Dz13 were suspended in 2013.
Sonophoresis also known as phonophoresis, is a method that utilizes ultrasound to enhance the delivery of topical medications through the stratum corneum, to the epidermis and dermis. Sonophoresis allows for the enhancement of the permeability of the skin along with other modalities, such as iontophoresis, to deliver drugs with lesser side effects. Currently, sonophoresis is used widely in transdermal drug delivery, but has potential applications in other sectors of drug delivery, such as the delivery of drugs to the eye and brain.
Transfersome is a proprietary drug delivery technology, an artificial vesicle designed to exhibit the characteristics of a cell vesicle suitable for controlled and potentially targeted drug delivery. Some evidence has shown efficacy for its use for drug delivery without causing skin irritation, potentially being used to treat skin cancer. Transfersome is made by the German company IDEA AG.
Isopropyl myristate (IPM) is the ester of isopropyl alcohol and myristic acid.
Skin absorption is a route by which substances can enter the body through the skin. Along with inhalation, ingestion and injection, dermal absorption is a route of exposure for toxic substances and route of administration for medication. Absorption of substances through the skin depends on a number of factors, the most important of which are concentration, duration of contact, solubility of medication, and physical condition of the skin and part of the body exposed.
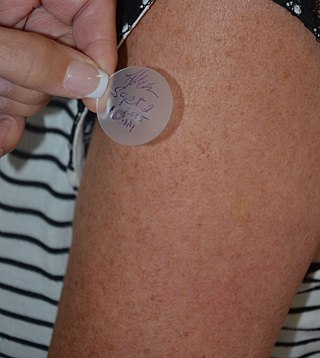
Transdermal is a route of administration wherein active ingredients are delivered across the skin for systemic distribution. Examples include transdermal patches used for medicine delivery. The drug is administered in the form of a patch or ointment that delivers the drug into the circulation for systemic effect.

Phonophoresis, also known as sonophoresis, is the method of using ultrasound waves to increase skin permeability in order to improve the effectiveness of transdermal drug delivery. This method intersects drug delivery and ultrasound therapy. By assisting transdermal drug delivery, phonophoresis can be a painless treatment and an alternative to a needle.
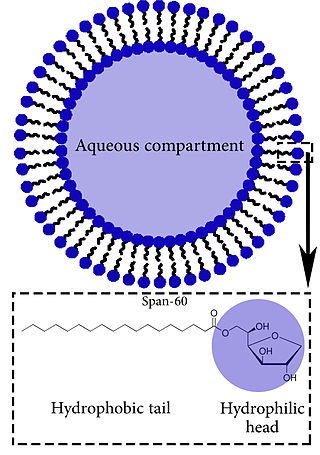
Niosomes are vesicles composed of non-ionic surfactants, incorporating cholesterol as an excipient. Niosomes are utilized for drug delivery to specific sites to achieve desired therapeutic effects. Structurally, niosomes are similar to liposomes as both consist of a lipid bilayer. However, niosomes are more stable than liposomes during formation processes and storage. Niosomes trap hydrophilic and lipophilic drugs, either in an aqueous compartment or in a vesicular membrane compartment composed of lipid material.
DDAIP is a pharmaceutical ingredient added to topical products to increase penetration through the skin. Chemically, DDAIP is an ester of N,N-dimethylalanine and dodecanol, although as of now the structural formula shows an ester with decanol (C10) instead. DDAIP is typically formulated as its hydrochloride salt (DDAIP.HCl). This salt is a white crystalline solid with a melting range of 88-93 °C and is an amphiphilic molecule with a pKa of 4.87 that is soluble in water up to about 40% w/v. DDAIP is proprietary to NexMed USA, a subsidiary of Apricus Biosciences.
A vesosome is a multi-compartmental structure of lipidic nature used to deliver drugs. They can be considered multivesicular vesicles (MVV) and are, therefore, liposome-derived structures.

Topical cream formulation is an emulsion semisolid dosage form that is used for skin external application. Most of the topical cream formulations contain more than 20 per cent of water and volatiles and/or less than 50 per cent of hydrocarbons, waxes, or polyethylene glycols as the vehicle for external skin application. In a topical cream formulation, ingredients are dissolved or dispersed in either a water-in-oil (W/O) emulsion or an oil-in-water (O/W) emulsion. The topical cream formulation has a higher content of oily substance than gel, but a lower content of oily ingredient than ointment. Therefore, the viscosity of topical cream formulation lies between gel and ointment. The pharmacological effect of the topical cream formulation is confined to the skin surface or within the skin. Topical cream formulation penetrates through the skin by transcellular route, intercellular route, or trans-appendageal route. Topical cream formulation is used for a wide range of diseases and conditions, including atopic dermatitis (eczema), psoriasis, skin infection, acne, and wart. Excipients found in a topical cream formulation include thickeners, emulsifying agents, preservatives, antioxidants, and buffer agents. Steps required to manufacture a topical cream formulation include excipient dissolution, phase mixing, introduction of active substances, and homogenization of the product mixture.
Topical drug delivery (TDD) is a route of drug administration that allows the topical formulation to be delivered across the skin upon application, hence producing a localized effect to treat skin disorders like eczema. The formulation of topical drugs can be classified into corticosteroids, antibiotics, antiseptics, and anti-fungal. The mechanism of topical delivery includes the diffusion and metabolism of drugs in the skin. Historically, topical route was the first route of medication used to deliver drugs in humans in ancient Egyptian and Babylonian in 3000 BCE. In these ancient cities, topical medications like ointments and potions were used on the skin. The delivery of topical drugs needs to pass through multiple skin layers and undergo pharmacokinetics, hence factor like dermal diseases minimize the bioavailability of the topical drugs. The wide use of topical drugs leads to the advancement in topical drug delivery. These advancements are used to enhance the delivery of topical medications to the skin by using chemical and physical agents. For chemical agents, carriers like liposomes and nanotechnologies are used to enhance the absorption of topical drugs. On the other hand, physical agents, like micro-needles is other approach for enhancement ofabsorption. Besides using carriers, other factors such as pH, lipophilicity, and drug molecule size govern the effectiveness of topical formulation.
Penetration enhancers are chemical compounds that can facilitate the penetration of active pharmaceutical ingredients (API) into or through the poorly permeable biological membranes. These compounds are used in some pharmaceutical formulations to enhance the penetration of APIs in transdermal drug delivery and transmucosal drug delivery. They typically penetrate into the biological membranes and reversibly decrease their barrier properties.
A caffeine patch is a type of a transdermal patch designed to deliver caffeine to the body through the skin. The concept is similar to that of a nicotine patch.
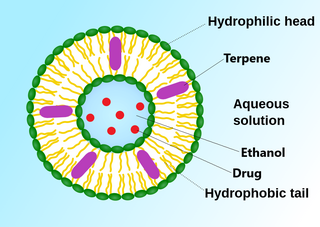
An invasome are a type of artificial vesicle nanocarrier that transport substances through the skin, the most superficial biological barrier. Vesicles are small particles surrounded by a lipid layer that can carry substances into and out of the cell. Artificial vesicles can be engineered to deliver drugs within the cell, with specific applications within transdermal drug delivery. However, the skin proves to be a barrier to effective penetration and delivery of drug therapies. Thus, invasomes are a new generation of vesicle with added structural components to assist with skin penetration.
Laser-assisted drug delivery (LADD) is a drug delivery technique commonly used in the dermatology field that involves lasers. As skin acts as a protective barrier to the environment, the absorption of topical products through the epidermis is limited; thus, different drug delivery modalities have been employed to improve the efficacy of these treatments. The use of lasers in LADD has been shown to enhance the penetration of drugs transdermal, leading to a higher absorption rate, limited systemic effects, and reduced duration of treatment. Although this technique has evolved in the past decade due to its efficacy through scientific research and clinical practice, there remain some limitations regarding the safety aspect that needs to be taken into consideration.









