A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and plus (+) and minus (−) signs. These are limited to a single typographic line of symbols, which may include subscripts and superscripts. A chemical formula is not a chemical name, and it contains no words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulas can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than are chemical names and structural formulas.
In chemistry, the empirical formula of a chemical compound is the simplest positive integer ratio of atoms present in a compound. A simple example of this concept is that the empirical formula of sulphur monoxide, or SO, would simply be SO, as is the empirical formula of disulfur dioxide, S2O2. This means that sulfur monoxide and disulfur dioxide, both compounds of sulfur and oxygen, will have the same empirical formula. However, their molecular formulas, which express the number of atoms in each molecule of a chemical compound, may not be the same.

A hydrogen bond is a partially electrostatic force of attraction between a hydrogen (H) atom which is bound to a more electronegative atom or group, such as nitrogen (N), oxygen (O), or fluorine (F)—the hydrogen bond donor—and another adjacent atom bearing a lone pair of electrons—the hydrogen bond acceptor. Weak hydrogen bonds occur even with C-H groups as donor

Stoichiometry is the calculation of reactants and products in chemical reactions.
In chemistry, the molar massM is a physical property defined as the mass of a given substance divided by the amount of substance. The base SI unit for molar mass is kg/mol. However, for historical reasons, molar masses are almost always expressed in g/mol.

The hydroxyl radical, •OH, is the neutral form of the hydroxide ion (OH−). Hydroxyl radicals are highly reactive (easily becoming hydroxyl groups) and consequently short-lived; however, they form an important part of radical chemistry. Most notably hydroxyl radicals are produced from the decomposition of hydroperoxides (ROOH) or, in atmospheric chemistry, by the reaction of excited atomic oxygen with water. It is also an important radical formed in radiation chemistry, since it leads to the formation of hydrogen peroxide and oxygen, which can enhance corrosion and SCC in coolant systems subjected to radioactive environments. Hydroxyl radicals are also produced during UV-light dissociation of H2O2 (suggested in 1879) and likely in Fenton chemistry, where trace amounts of reduced transition metals catalyze peroxide-mediated oxidations of organic compounds.

The trihydrogen cation, also known as protonated molecular hydrogen or H+
3, is one of the most abundant ions in the universe. It is stable in the interstellar medium (ISM) due to the low temperature and low density of interstellar space. The role that H+
3 plays in the gas-phase chemistry of the ISM is unparalleled by any other molecular ion. The cation is also the simplest triatomic molecule, since its two electrons are the only valence electrons in the system. It is also the simplest example of a three-center two-electron bond system.

An astrophysical maser is a naturally occurring source of stimulated spectral line emission, typically in the microwave portion of the electromagnetic spectrum. This emission may arise in molecular clouds, comets, planetary atmospheres, stellar atmospheres, or various other conditions in interstellar space.
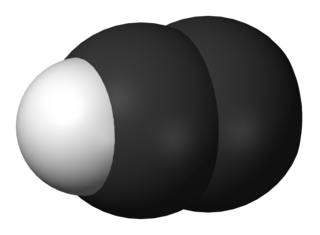
The ethynyl radical is an organic compound with the chemical formula C≡CH. It is a simple molecule that does not occur naturally on Earth but is abundant in the interstellar medium. It was first observed by electron spin resonance isolated in a solid argon matrix at liquid helium temperatures in 1963 by Cochran and coworkers at the Johns Hopkins Applied Physics Laboratory. It was first observed in the gas phase by Tucker and coworkers in November 1973 toward the Orion Nebula, using the NRAO 11m radio telescope. It has since been detected in a large variety of interstellar environments, including dense molecular clouds, bok globules, star forming regions, the shells around carbon-rich evolved stars, and even in other galaxies.

The hydrohelium(1+) cation, HeH+, also known as the helium hydride ion or helium-hydride molecular ion, is a positively charged ion formed by the reaction of a proton with a helium atom in the gas phase, first produced in the laboratory in 1925. It is isoelectronic with molecular hydrogen. It is the strongest known acid, with a proton affinity of 177.8 kJ/mol. It has been suggested that HeH+ should occur naturally in the interstellar medium, but it has not yet been detected. It is the simplest heteronuclear ion, and is comparable with the hydrogen molecular ion, H+
2. Unlike H+
2, however, it has a permanent dipole moment, which makes its spectroscopic characterization easier. The calculated dipole moment of HeH+ is 2.26 or 2.84.
Propynylidyne is a chemical compound that has been identified in interstellar space.
Interstellar formaldehyde (a topic relevant to astrochemistry) was first discovered in 1969 by L. Snyder et al. using the National Radio Astronomy Observatory. Formaldehyde (H2CO) was detected by means of the 111 - 110 ground state rotational transition at 4830 MHz. On 11 August 2014, astronomers released studies, using the Atacama Large Millimeter/Submillimeter Array (ALMA) for the first time, that detailed the distribution of HCN, HNC, H2CO, and dust inside the comae of comets C/2012 F6 (Lemmon) and C/2012 S1 (ISON).
Cyanopolyynes are a group of chemicals with the chemical formula HC
nN. Structurally, they are polyynes with a cyano group covalently bonded to one of the terminal acetylene units. A rarely seen group of molecules both due to the difficulty in production and the unstable nature of the paired groups, the cyanopolyynes have been observed as a major organic component in interstellar clouds. This is believed to be due to the hydrogen scarcity of some of these clouds. Interference with hydrogen is one of the reason for the molecule's instability due to the energetically favorable dissociation back into hydrogen cyanide and acetylene. Cyanopolyynes were first discovered in interstellar molecular clouds in 1971 using millimeter wave and microwave telescopes. Since then many higher weight cyanopolyynes such as HC
7N and HC
11N have been discovered, although some of these identifications have been disputed. Other derivatives such as methylcyanoacetylene CH
3C
3N and ethylcyanoacetylene CH
3CH
2C
3N have been observed as well. The simplest example is cyanoacetylene, H−C≡C−C≡N. Cyanoacetylene is more common on earth and it is believed to be the initial reagent for most of the photo-catalyzed formation of the interstellar cyanopolyynes. Cyanoacetylene is one of the molecules that was produced in the Miller–Urey experiment and is expected to be found in carbon-rich environments.

Chromium(I) hydride, systematically named chromium hydride, is an inorganic compound with the chemical formula (CrH)
n. It occurs naturally in some kinds of stars where it has been detected by its spectrum. However, molecular chromium(I) hydride with the formula CrH has been isolated in solid gas matrices. The molecular hydride is very reactive. As such the compound is not well characterised, although many of its properties have been calculated via computational chemistry.

Iron(I) hydride, systematically named iron hydride and poly(hydridoiron) is a solid inorganic compound with the chemical formula (FeH)
n. It is both thermodynamically and kinetically unstable toward decomposition at ambient temperature, and as such, it little is known about its bulk properties.
Magnesium monohydride is a molecular gas with formula MgH that exists at high temperatures, such as the atmospheres of the Sun and stars. It was originally known as magnesium hydride, although that name is now more commonly used when referring to the similar chemical magnesium dihydride.
Argonium, an ion combining a proton and an argon atom (and so also called protonated argon), (ArH+) can be made in an electric discharge, and was the first noble gas molecular ion to be found in interstellar space.

Tricarbon monoxide C3O is a reactive radical oxocarbon molecule found in space, and which can be made as a transient substance in the laboratory. It can be trapped in an inert gas matrix or made as a short lived gas. C3O can be classified as a ketene or an oxocumulene a kind of heterocumulene.
Silicon monohydride is a chemical substance occurring as a molecule found in stars and probably existing in interstellar space, or as a monolayer on the surface of solid silicon. The SiH molecule is a radical, and can be made experimentally by striking an electric arc to silicon on a low pressure hydrogen gas.












