Related Research Articles

Amino acids are organic compounds that contain amine (–NH2) and carboxyl (–COOH) functional groups, along with a side chain (R group) specific to each amino acid. The key elements of an amino acid are carbon (C), hydrogen (H), oxygen (O), and nitrogen (N), although other elements are found in the side chains of certain amino acids. About 500 naturally occurring amino acids are known (though only 20 appear in the genetic code) and can be classified in many ways. They can be classified according to the core structural functional groups' locations as alpha- (α-), beta- (β-), gamma- (γ-) or delta- (δ-) amino acids; other categories relate to polarity, pH level, and side chain group type (aliphatic, acyclic, aromatic, containing hydroxyl or sulfur, etc.). In the form of proteins, amino acid residues form the second-largest component (water is the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis.
Collagen is the main structural protein in the extracellular matrix found in the body's various connective tissues. As the main component of connective tissue, it is the most abundant protein in mammals, making up from 25% to 35% of the whole-body protein content. Collagen consists of amino acids bound together to form a triple helix of elongated fibril known as a collagen helix. It is mostly found in connective tissue such as cartilage, bones, tendons, ligaments, and skin.

A cell wall is a structural layer surrounding some types of cells, just outside the cell membrane. It can be tough, flexible, and sometimes rigid. It provides the cell with both structural support and protection, and also acts as a filtering mechanism. Cell walls are present in most prokaryotes, in algae, fungi and eukaryotes including plants but are absent in animals. A major function is to act as pressure vessels, preventing over-expansion of the cell when water enters.

Protein primary structure is the linear sequence of amino acids in a peptide or protein. By convention, the primary structure of a protein is reported starting from the amino-terminal (N) end to the carboxyl-terminal (C) end. Protein biosynthesis is most commonly performed by ribosomes in cells. Peptides can also be synthesized in the laboratory. Protein primary structures can be directly sequenced, or inferred from DNA sequences.

Proline (symbol Pro or P) is an organic acid classed as a proteinogenic amino acid (used in the biosynthesis of proteins), although it does not contain the amino group -NH
2 but is rather a secondary amine. The secondary amine nitrogen is in the protonated NH2+ form under biological conditions, while the carboxy group is in the deprotonated −COO− form. The "side chain" from the α carbon connects to the nitrogen forming a pyrrolidine loop, classifying it as a aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it from the non-essential amino acid L-glutamate. It is encoded by all the codons starting with CC (CCU, CCC, CCA, and CCG).

Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula HOOC-CH-(NH2)-CH2-SH. The thiol side chain in cysteine often participates in enzymatic reactions, as a nucleophile. The thiol is susceptible to oxidation to give the disulfide derivative cystine, which serves an important structural role in many proteins. When used as a food additive, it has the E number E920. It is encoded by the codons UGU and UGC.

(2S,4R)-4-Hydroxyproline, or L-hydroxyproline (C5H9O3N), is an amino acid, abbreviated as Hyp or O, e.g., in Protein Data Bank.
Hydroxylation is a chemical process that introduces a hydroxyl group (-OH) into an organic compound. In biochemistry, hydroxylation reactions are often facilitated by enzymes called hydroxylases. Hydroxylation is the first step in the oxidative degradation of organic compounds in air. It is extremely important in detoxification since hydroxylation converts lipophilic compounds into water-soluble (hydrophilic) products that are more readily removed by the kidneys or liver and excreted. Some drugs are activated or deactivated by hydroxylation.

Proteinogenic amino acids are amino acids that are incorporated biosynthetically into proteins during translation. The word "proteinogenic" means "protein creating". Throughout known life, there are 22 genetically encoded (proteinogenic) amino acids, 20 in the standard genetic code and an additional 2 that can be incorporated by special translation mechanisms.
An oligosaccharide is a saccharide polymer containing a small number of monosaccharides. Oligosaccharides can have many functions including cell recognition and cell binding. For example, glycolipids have an important role in the immune response.
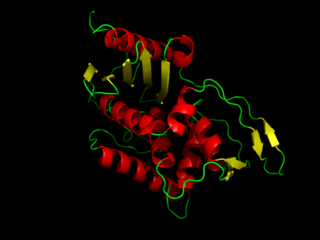
DD-transpeptidase is a bacterial enzyme that catalyzes the transfer of the R-L-aca-D-alanyl moiety of R-L-aca-D-alanyl-D-alanine carbonyl donors to the γ-OH of their active-site serine and from this to a final acceptor. It is involved in bacterial cell wall biosynthesis, namely, the transpeptidation that crosslinks the peptide side chains of peptidoglycan strands.

Resilin is an elastomeric protein found in many insects and other arthropods. It provides soft rubber-elasticity to mechanically active organs and tissue; for example, it enables insects of many species to jump or pivot their wings efficiently. Resilin was first discovered by Torkel Weis-Fogh in locust wing-hinges.
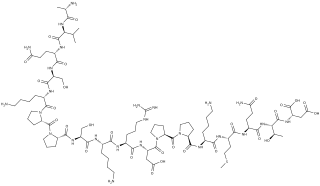
Systemin is a plant peptide hormone involved in the wound response in the family Solanaceae. It was the first plant hormone that was proven to be a peptide having been isolated from tomato leaves in 1991 by a group led by Clarence A. Ryan. Since then, other peptides with similar functions have been identified in tomato and outside of the Solanaceae. Hydroxyproline-rich glycopeptides were found in tobacco in 2001 and AtPEPs were found in Arabidopsis thaliana in 2006. Their precursors are found both in the cytoplasm and cell walls of plant cells, upon insect damage, the precursors are processed to produce one or more mature peptides. The receptor for systemin was first thought to be the same as the brassinolide receptor but this is now uncertain. The signal transduction processes that occur after the peptides bind are similar to the cytokine-mediated inflammatory immune response in animals. Early experiments showed that systemin travelled around the plant after insects had damaged the plant, activating systemic acquired resistance, now it is thought that it increases the production of jasmonic acid causing the same result. The main function of systemins is to coordinate defensive responses against insect herbivores but they also affect plant development. Systemin induces the production of protease inhibitors which protect against insect herbivores, other peptides activate defensins and modify root growth. They have also been shown to affect plants' responses to salt stress and UV radiation. AtPEPs have been shown to affect resistance against oomycetes and may allow A. thaliana to distinguish between different pathogens. In Nicotiana attenuata, some of the peptides have stopped being involved in defensive roles and instead affect flower morphology.

Procollagen-proline dioxygenase, commonly known as prolyl hydroxylase, is a member of the class of enzymes known as alpha-ketoglutarate-dependent hydroxylases. These enzymes catalyze the incorporation of oxygen into organic substrates through a mechanism that requires alpha-Ketoglutaric acid, Fe2+, and ascorbate. This particular enzyme catalyzes the formation of (2S, 4R)-4-hydroxyproline, a compound that represents the most prevalent post-translational modification in the human proteome.

T-cell surface glycoprotein CD3 gamma chain is a protein that in humans is encoded by the CD3G gene.
Glycopeptides are peptides that contain carbohydrate moieties (glycans) covalently attached to the side chains of the amino acid residues that constitute the peptide.
Arabinogalactan-proteins (AGP) are members of the hydroxyproline (Hyp)-rich cell wall glycoprotein superfamily, and are extensively glycosylated, meaning that each one consists of a protein molecule attached to sugar molecules.

In molecular biology, the protein domain SdrG C terminal refers to the C terminus domain of an adhesin found only on the cell walls of bacteria. More specifically, SdrG is only found in gram-positive bacteria. This particular domain binds to a glycoprotein named fibrinogen. SdrG stands for serine-aspartate dipeptide repeats, which as its name suggests, contains repeats of two amino acids, serine and aspartate.

In biochemistry, non-coded or non-proteinogenic amino acids are those not naturally encoded or found in the genetic code of any organism. Despite the use of only 22 amino acids by the translational machinery to assemble proteins, over 140 amino acids are known to occur naturally in proteins and thousands more may occur in nature or be synthesized in the laboratory. Many non-proteinogenic amino acids are noteworthy because they are;

Zingibain, zingipain, or ginger protease is a cysteine protease enzyme found in ginger rhizomes. It catalyses the preferential cleavage of peptides with a proline residue at the P2 position. It has two distinct forms, ginger protease I (GP-I) and ginger protease II (GP-II).
References
- ↑ Lamport, D.T.A. (1965) Advances in Botanical Research 2:151-218 The protein component of primary cell walls
- ↑ Lamport,D.T.A.; Northcote,D.H. (1960) Nature 188:665-666 Hydroxyproline in primary cell walls of higher plants
- ↑ Rapaport,H. (2006) Ordered peptide assemblies at interfaces. Supramolecular Chemistry, 18, 445-454.
- 1 2 Lamport,D.T.A. (1963) Oxygen fixation into hydroxyproline of plant cell wall protein. J.Biol.Chem., 238, 1438-1440.
- ↑ Lamport,D.T.A. (1973): The glycopeptide linkages of extensin, O-D-galactosyl serine and O-L-arabinosyl hydroxyproline. In: Biogenesis of plant cell wall polysaccharides, Anonymouspp. 149-164. Academic Press Inc., New York.
- ↑ Lamport,D.T.A. (1977): Structure, biosynthesis and significance of cell wall glycoproteins. In: Recent Advances in Phytochemistry, edited by F.A.Loewus, et al, pp. 79-115. Plenum Publishing Corp., New York.
- ↑ Fong,C., Kieliszewski,M.J., de Zacks,R., Leykam,J.F., and Lamport,D.T.A. (1992) A gymnosperm extensin contains the serine-tetrahydroxyproline motif. Plant Physiol., 99, 548-552.
- ↑ Lamport,D.T.A. (1967) Hydroxyproline-O-glycosidic linkage of the plant cell wall glycoprotein extensin. Nature, 216, 1322-1324.
- ↑ van Holst,G.-J. and Varner,J.E. (1984) Reinforced polyproline II conformation in a hydroxyproline-rich glycoprotein from carrot root. Plant Physiol., 74, 247-251.
- ↑ Lamport,D.T.A., Katona,L., and Roerig,S. (1973) Galactosyl serine in extensin. Biochem.J., 133, 125-131.
- ↑ Epstein,L. and Lamport,D.T.A. (1984) An intramolecular linkage involving isodityrosine in extensin. Phytochemistry, 23, 1241-1246.
- ↑ Everdeen,D.S., Kiefer,S., Willard,J.J., Muldoon,E.P., Dey,P.M., Li,X.-B., and Lamport,D.T.A. (1988) Enzymic crosslinkage of monomeric extensin precursors in vitro. Plant Physiol., 87, 616-621.
- ↑ Lamport,D.T.A. (1989): Extensin peroxidase ties the knots in the extensin network. In: Cell Separation in Plants, edited by D.J. Osborne, et al, pp. 101-113. Springer-Verlag, Berlin.
- ↑ Schnabelrauch,L.S., Kieliszewski,M.J., Upham,B.L., Alizedeh,H., and Lamport,D.T.A. (1996) Isolation of pI 4.6 extensin peroxidase from tomato cell suspension cultures and identification of Val-Tyr-Lys as putative intermolecular cross-link site. Plant J., 9, 477-489.