
Stainless steel, also known as inox, corrosion-resistant steel (CRES), and rustless steel, is an iron-based alloy containing a minimum level of chromium that is resistant to rusting and corrosion. Stainless steel's resistance to corrosion results from the 10.5%, or more, chromium content which forms a passive film that can protect the material and self-heal in the presence of oxygen. It can also be alloyed with other elements such as molybdenum, carbon, nickel and nitrogen to develop a range of different properties depending on its specific use.

Martensitic stainless steels are a family of stainless steels having body-centered tetragonal (BCT) crystal structure and a predominately martensite structure. They are characterized by being magnetic and having the ability to be hardened through heat treatment. Martensitic stainless steels are designated as part of the 400-series of stainless steels in the SAE steel grades numbering system.

Carbon steel is a steel with carbon content from about 0.05 up to 2.1 percent by weight. The definition of carbon steel from the American Iron and Steel Institute (AISI) states:
AL-6XN is a type of weldable stainless steel that consist of an alloy of nickel (24%), chromium (22%) and molybdenum (6.3%) with other trace elements such as nitrogen.
The weldability, also known as joinability, of a material refers to its ability to be welded. Many metals and thermoplastics can be welded, but some are easier to weld than others. A material's weldability is used to determine the welding process and to compare the final weld quality to other materials.
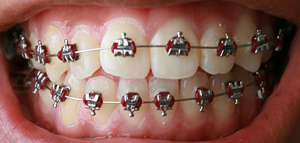
An archwire in orthodontics is a wire conforming to the alveolar or dental arch that can be used with dental braces as a source of force in correcting irregularities in the position of the teeth. An archwire can also be used to maintain existing dental positions; in this case it has a retentive purpose.
Marine grade stainless alloys typically contain molybdenum to resist the corrosive effects of NaCl or salt in seawater. Concentrations of salt in seawater can vary, and splash zones can cause concentrations to increase dramatically from the spray and evaporation.

In materials science, intergranular corrosion (IGC), also known as intergranular attack (IGA), is a form of corrosion where the boundaries of crystallites of the material are more susceptible to corrosion than their insides.
Austenitic stainless steel is one of the five families of stainless steel. Its primary crystalline structure is austenite. Such steels are not hardenable by heat treatment and are essentially non-magnetic. This structure is achieved by adding enough austenite-stabilizing elements such as nickel, manganese and nitrogen. The Incoloy family of alloys belong to the category of super austenitic stainless steels.

The SAE steel grades system is a standard alloy numbering system for steel grades maintained by SAE International.

Alloy steel is steel that is alloyed with a variety of elements in amounts between 1.0% and 50% by weight, typically to improve its mechanical properties.
Zeron 100 is a super duplex stainless steel developed by Rolled Alloys. The alloy has excellent corrosion resistance combined with high strength. It typically contains 25% chromium and 7% nickel and 3.6% molybdenum along with copper and tungsten additions. Zeron 100 has a 50–50 austenitic–ferritic structure. It also has greater resistance to chloride pitting, crevice corrosion and stress corrosion cracking than exhibited by the standard 300 series stainless steels.
Incoloy refers to a range of superalloys now produced by the Special Metals Corporation (SMC) group of companies and created with a trademark by the Inco company in 1952. Originally Inco protected these alloys by patent. In 2000, the SMC published a 61-page document entitled "High-Performance Alloys for Resistance to Aqueous Corrosion" highlighting Incoloy, as well as Monel and Inconel products, and their use in fluid environments such as sulfuric acid, hydrochloric acid, hydrofluoric acid, phosphoric acid, nitric acid, other acids as well as freshwater environments.
SAF 2205, is a Alleima-owned trademark for a 22Cr duplex (ferritic-austenitic) stainless steel. SAF derives from Sandvik Austenite Ferrite. The nominal chemical composition of SAF 2205 is 22% chromium, 5% nickel, 3.2% molybdenum and other alloying elements such as nitrogen and manganese. The UNS designation for SAF 2205 is S31803/S32205 and the EN steel no. is 1.4462. SAF 2205 or Duplex 2205 is often used as an alternative to expensive 904L stainless steel owing to similar properties but cheaper ingredients. Duplex stainless steel is available in multiple forms like bars, billets, pipes, tubes, sheets, plates and even processed to fittings and flanges.
SAF 2507, is a Alleima-owned trademark for a 25Cr duplex (ferritic-austenitic) stainless steel. The nominal chemical composition of SAF 2507 is 25% chromium, 7% nickel, 4% molybdenum and other alloying elements such as nitrogen and manganese. The UNS designation for SAF 2507 is S32750 and the EN steel no. is 1.4410. SAF derives from Sandvik Austenite Ferrite.
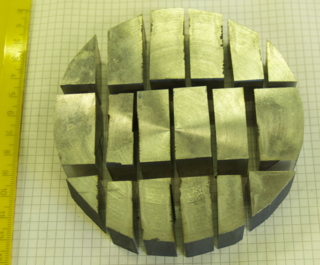
Duplex stainless steels are a family of stainless steels. These are called duplex grades because their metallurgical structure consists of two phases, austenite and ferrite in roughly equal proportions.

SAE 304 stainless steel is the most common stainless steel. It is an alloy of iron, carbon, chromium and nickel. It is an austenitic stainless steel, and is therefore not magnetic. It is less electrically and thermally conductive than carbon steel. It has a higher corrosion resistance than regular steel and is widely used because of the ease in which it is formed into various shapes.
904L is an austenitic stainless steel. It is softer than 316L, and its molybdenum addition gives it superior resistance to localized attack by chlorides and greater resistance reducing acids; in particular, its copper addition gives it useful corrosion resistance to all concentrations of sulfuric acid. Its high alloying content also gives it greater resistance to chloride stress corrosion cracking, but it is still susceptible. Its low carbon content makes it resistant to sensitization by welding and prevents intergranular corrosion.

Duplex stainless steels are a family of alloys with a two-phase microstructure consisting of both austenitic and ferritic phases. They offer excellent mechanical properties, corrosion resistance, and toughness compared to other types of stainless steel. However, duplex stainless steel can be susceptible to a phenomenon known as 475 °C (887 °F) embrittlement or duplex stainless steel age hardening, which is a type of aging process that causes loss of plasticity in duplex stainless steel when it is heated in the range of 250 to 550 °C. At this temperature range, spontaneous phase separation of the ferrite phase into iron-rich and chromium-rich nanophases occurs, with no change in the mechanical properties of the austenite phase. This type of embrittlement is due to precipitation hardening, which makes the material become brittle and prone to cracking.










