Piezoelectricity is the electric charge that accumulates in certain solid materials in response to applied mechanical stress. The word piezoelectricity means electricity resulting from pressure and latent heat. It is derived from the Greek word πιέζειν; piezein, which means to squeeze or press, and ἤλεκτρον ēlektron, which means amber, an ancient source of electric charge. French physicists Jacques and Pierre Curie discovered piezoelectricity in 1880.
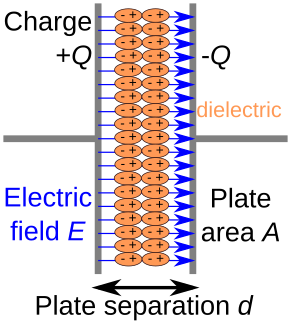
A dielectric is an electrical insulator that can be polarized by an applied electric field. When a dielectric is placed in an electric field, electric charges do not flow through the material as they do in an electrical conductor but only slightly shift from their average equilibrium positions causing dielectric polarization. Because of dielectric polarization, positive charges are displaced in the direction of the field and negative charges shift in the opposite direction. This creates an internal electric field that reduces the overall field within the dielectric itself. If a dielectric is composed of weakly bonded molecules, those molecules not only become polarized, but also reorient so that their symmetry axes align to the field.
Ferroelectricity is a characteristic of certain materials that have a spontaneous electric polarization that can be reversed by the application of an external electric field. All ferroelectrics are pyroelectric, with the additional property that their natural electrical polarization is reversible. The term is used in analogy to ferromagnetism, in which a material exhibits a permanent magnetic moment. Ferromagnetism was already known when ferroelectricity was discovered in 1920 in Rochelle salt by Valasek. Thus, the prefix ferro, meaning iron, was used to describe the property despite the fact that most ferroelectric materials do not contain iron. Materials that are both ferroelectric and ferromagnetic are known as multiferroics.
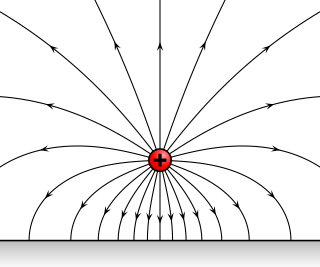
Pyroelectricity is a property of certain crystals which are naturally electrically polarized and as a result contain large electric fields. Pyroelectricity can be described as the ability of certain materials to generate a temporary voltage when they are heated or cooled. The change in temperature modifies the positions of the atoms slightly within the crystal structure, such that the polarization of the material changes. This polarization change gives rise to a voltage across the crystal. If the temperature stays constant at its new value, the pyroelectric voltage gradually disappears due to leakage current.
An electret is a dielectric material that has a quasi-permanent electric charge or dipole polarisation. An electret generates internal and external electric fields, and is the electrostatic equivalent of a permanent magnet. Although Oliver Heaviside coined this term in 1885, materials with electret properties were already known to science and had been studied since the early 1700s. One particular example is the electrophorus, a device consisting of a slab with electret properties and a separate metal plate. The electrophorus was originally invented by Johan Carl Wilcke in Sweden and again by Alessandro Volta in Italy.
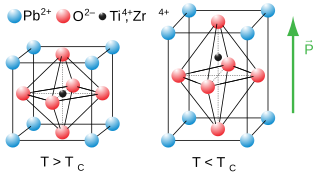
Lead zirconate titanate is an inorganic compound with the chemical formula Pb[ZrxTi1−x]O3 (0≤x≤1). Also called PZT, it is a ceramic perovskite material that shows a marked piezoelectric effect, meaning that the compound changes shape when an electric field is applied. It is used in a number of practical applications such as ultrasonic transducers and piezoelectric resonators. It is a white to off-white solid.
Electrostriction is a property of all electrical non-conductors, or dielectrics, that causes them to change their shape under the application of an electric field.
Energy harvesting is the process by which energy is derived from external sources, captured, and stored for small, wireless autonomous devices, like those used in wearable electronics and wireless sensor networks.
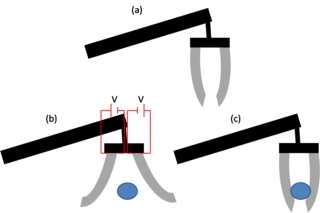
Electroactive polymers, or EAPs, are polymers that exhibit a change in size or shape when stimulated by an electric field. The most common applications of this type of material are in actuators and sensors. A typical characteristic property of an EAP is that they will undergo a large amount of deformation while sustaining large forces.
Multiferroics are defined as materials that exhibit more than one of the primary ferroic properties:

Barium titanate is an inorganic compound with chemical formula BaTiO3. Barium titanate appears white as a powder and is transparent when prepared as large crystals. It is a ferroelectric ceramic material that exhibits the photorefractive effect and piezoelectric properties. It is used in capacitors, electromechanical transducers and nonlinear optics.
Ferroelectrets also known as piezoelectrets, are thin films of polymer foams, exhibiting piezoelectric and pyroelectric properties after electric charging. Ferroelectret foams usually consist of a cellular polymer structure filled with air. Polymer-air composites are elastically soft due to their high air content as well as due to the size and shape of the polymer walls. Their elastically soft composite structure is one essential key for the working principle of ferroelectrets, besides the permanent trapping of electric charges inside the polymer voids. The elastic properties allow large deformations of the electrically charged voids. However, the composite structure can also possibly limit the stability and consequently the range of applications.
A polymer blend, or polymer mixture, is a member of a class of materials analogous to metal alloys, in which at least two polymers are blended together to create a new material with different physical properties.

Electron-beam processing or electron irradiation (EBI) is a process that involves using electrons, usually of high energy, to treat an object for a variety of purposes. This may take place under elevated temperatures and nitrogen atmosphere. Possible uses for electron irradiation include sterilization and cross-linking of polymers.
Qiming Zhang is a distinguished professor of Electrical Engineering and Materials Science and Engineering at Pennsylvania State University. He is also the vice President & CTO at Strategic Polymer Sciences, Inc.
A Nanogenerator is a type of technology that converts mechanical/thermal energy as produced by small-scale physical change into electricity. A Nanogenerator has three typical approaches: piezoelectric, triboelectric, and pyroelectric nanogenerators. Both the piezoelectric and triboelectric nanogenerators can convert mechanical energy into electricity. However, pyroelectric nanogenerators can be used to harvest thermal energy from a time-dependent temperature fluctuation.
A biotransducer is the recognition-transduction component of a biosensor system. It consists of two intimately coupled parts; a bio-recognition layer and a physicochemical transducer, which acting together converts a biochemical signal to an electronic or optical signal. The bio-recognition layer typically contains an enzyme or another binding protein such as antibody. However, oligonucleotide sequences, sub-cellular fragments such as organelles and receptor carrying fragments, single whole cells, small numbers of cells on synthetic scaffolds, or thin slices of animal or plant tissues, may also comprise the bio-recognition layer. It gives the biosensor selectivity and specificity. The physicochemical transducer is typically in intimate and controlled contact with the recognition layer. As a result of the presence and biochemical action of the analyte, a physico-chemical change is produced within the biorecognition layer that is measured by the physicochemical transducer producing a signal that is proportionate to the concentration of the analyte. The physicochemical transducer may be electrochemical, optical, electronic, gravimetric, pyroelectric or piezoelectric. Based on the type of biotransducer, biosensors can be classified as shown to the right.
Piezoelectric Micromachined Ultrasonic Transducers (PMUT) are MEMS-based piezoelectric ultrasonic transducers. Unlike bulk piezoelectric transducers which use the thickness-mode motion of a plate of piezoelectric ceramic such as PZT or single-crystal PMN-PT, PMUT are based on the flexural motion of a thin membrane coupled with a thin piezoelectric film, such as PVDF. In comparison with bulk piezoelectric ultrasound transducers, PMUT can offer advantages such as increased bandwidth, flexible geometries, natural acoustic impedance match with water, reduced voltage requirements, mixing of different resonant frequencies and potential for integration with supporting electronic circuits especially for miniaturized high frequency applications.
A polar metal, metallic ferroeletric, or ferroelectric metal is a metal that contains an electric dipole moment. Its components have an ordered electric dipole. Such metals should be unexpected, because the charge should conduct by way of the free electrons in the metal and neutralize the polarized charge. However they do exist. Probably the first report of a polar metal was in single crystals of the cuprate superconductors YBa2Cu3O7-δ,. A polarization was observed along one (001) axis by pyroelectric effect measurements, and the sign of the polarization was shown to be reversible, while its magnitude could be increased by poling by an electric field. The polarization was found to disappear in the superconducting state.. The lattice distortions responsible were considered to be a result of oxygen ion displacements induced by doped charges that break inversion symmetry. The effect was utilized for fabrication of pyroelectric detectors for space applications, having the advantage of large pyroelectric coefficient and low intrinsic resistance. Another substance family that can produce a polar metal is the nickelate perovskites. One example interpreted to show polar metallic behavior is lanthanum nickelate, LaNiO3. A thin film of LaNiO3 grown on the (111) crystal face of lanthanum aluminate, (LaAlO3) was interpreted to be both conductor and a polar material at room temperature. The resistivity of this system, however, shows an upturn with decreasing temperature, hence does not strictly adhere to the definition of a metal. Also, when grown 3 or 4 unit cells thick (1-2 nm) on the (100) crystal face of LaAlO3, the LaNiO3 can be a polar insulator or polar metal depending on the atomic termination of the surface. Lithium osmate, LiOsO3 also undergoes a ferrorelectric transition when it is cooled below 140K. The point group changes from R3c to R3c losing its centrosymmetry. At room temperature and below, lithium osmate is an electric conductor, in single crystal, polycrystalline or powder forms, and the ferroelectric form only appears below 140K. Above 140K the material behaves like a normal metal.