Collagen is the main structural protein in the extracellular matrix found in the body's various connective tissues. As the main component of connective tissue, it is the most abundant protein in mammals, making up from 25% to 35% of the whole-body protein content. Collagen consists of amino acids bound together to form a triple helix of elongated fibril known as a collagen helix. It is mostly found in connective tissue such as cartilage, bones, tendons, ligaments, and skin.

Integrins are transmembrane receptors that facilitate cell-extracellular matrix (ECM) adhesion. Upon ligand binding, integrins activate signal transduction pathways that mediate cellular signals such as regulation of the cell cycle, organization of the intracellular cytoskeleton, and movement of new receptors to the cell membrane. The presence of integrins allows rapid and flexible responses to events at the cell surface.

A tendon or sinew is a tough band of fibrous connective tissue that connects muscle to bone and is capable of withstanding tension.

Fibronectin is a high-molecular weight (~440kDa) glycoprotein of the extracellular matrix that binds to membrane-spanning receptor proteins called integrins. Fibronectin also binds to other extracellular matrix proteins such as collagen, fibrin, and heparan sulfate proteoglycans.

In biology, the extracellular matrix (ECM) is a three-dimensional network of extracellular macromolecules, such as collagen, enzymes, and glycoproteins, that provide structural and biochemical support to surrounding cells. Because multicellularity evolved independently in different multicellular lineages, the composition of ECM varies between multicellular structures; however, cell adhesion, cell-to-cell communication and differentiation are common functions of the ECM.
In biology, matrix is the material in between a eukaryotic organism's cells.

The basement membrane is a thin, pliable sheet-like type of extracellular matrix, that provides cell and tissue support and acts as a platform for complex signalling. The basement membrane sits between epithelial tissues including mesothelium and endothelium, and the underlying connective tissue.
Cell adhesion molecules (CAMs) are a subset of cell adhesion proteins located on the cell surface involved in binding with other cells or with the extracellular matrix (ECM) in the process called cell adhesion. In essence, cell adhesion molecules help cells stick to each other and to their surroundings. Cell adhesion is a crucial component in maintaining tissue structure and function. In fully developed animals, these molecules play an integral role in creating force and movement and consequently ensure that organs are able to execute their functions. In addition to serving as "molecular glue", cell adhesion is important in affecting cellular mechanisms of growth, contact inhibition, and apoptosis. Oftentimes aberrant expression of CAMs will result in pathologies ranging from frostbite to cancer.

Fibrils are structural biological materials found in nearly all living organisms. Not to be confused with fibers or filaments, fibrils tend to have diameters ranging from 10-100 nanometers. Fibrils are not usually found alone but rather are parts of greater hierarchical structures commonly found in biological systems. Due to the prevalence of fibrils in biological systems, their study is of great importance in the fields of microbiology, biomechanics, and materials science.

A disintegrin and metalloproteinase with thrombospondin motifs 2 (ADAM-TS2) also known as procollagen I N-proteinase is an enzyme that in humans is encoded by the ADAMTS2 gene.

Collagen, type I, alpha 1, also known as alpha-1 type I collagen, is a protein that in humans is encoded by the COL1A1 gene. COL1A1 encodes the major component of type I collagen, the fibrillar collagen found in most connective tissues, including cartilage.
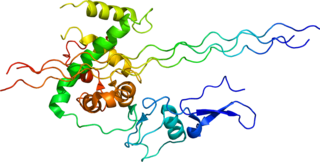
Type III Collagen is a homotrimer, or a protein composed of three identical peptide chains (monomers), each called an alpha 1 chain of type III collagen. Formally, the monomers are called collagen type III, alpha-1 chain and in humans are encoded by the COL3A1 gene. Type III collagen is one of the fibrillar collagens whose proteins have a long, inflexible, triple-helical domain.

Tenascins are extracellular matrix glycoproteins. They are abundant in the extracellular matrix of developing vertebrate embryos and they reappear around healing wounds and in the stroma of some tumors.
The mesohyl, formerly known as mesenchyme or as mesoglea, is the gelatinous matrix within a sponge. It fills the space between the external pinacoderm and the internal choanoderm. The mesohyl resembles a type of connective tissue and contains several amoeboid cells such as amebocytes, as well as fibrils and skeletal elements. For a long time, it has been largely accepted that sponges lack true tissue, but it is currently debated as to whether mesohyl and pinacoderm layers are tissues.
Type I collagen is the most abundant collagen of the human body. It forms large, eosinophilic fibers known as collagen fibers. It is present in scar tissue, the end product when tissue heals by repair, as well as tendons, ligaments, the endomysium of myofibrils, the organic part of bone, the dermis, the dentin, and organ capsules.

Collagen alpha-1(V) chain is a protein that in humans is encoded by the COL5A1 gene.

Tenascin C (TN-C) is a glycoprotein that in humans is encoded by the TNC gene. It is expressed in the extracellular matrix of various tissues during development, disease or injury, and in restricted neurogenic areas of the central nervous system. Tenascin-C is the founding member of the tenascin protein family. In the embryo it is made by migrating cells like the neural crest; it is also abundant in developing tendons, bone and cartilage.

Collagen alpha-3(V) chain is a protein that in humans is encoded by the COL5A3 gene.

Dermatopontin also known as tyrosine-rich acidic matrix protein (TRAMP) is a protein that in humans is encoded by the DPT gene. Dermatopontin is a 22-kDa protein of the noncollagenous extracellular matrix (ECM) estimated to comprise 12 mg/kg of wet dermis weight. To date, homologues have been identified in five different mammals and 12 different invertebrates with multiple functions. In vertebrates, the primary function of dermatopontin is a structural component of the ECM, cell adhesion, modulation of TGF-β activity and cellular quiescence). It also has pathological involvement in heart attacks and decreased expression in leiomyoma and fibrosis. In invertebrate, dermatopontin homologue plays a role in hemagglutination, cell-cell aggregation, and expression during parasite infection.
Feline cutaneous asthenia is a rare inheritable skin disease of cats characterised by abnormal elasticity, stretching, and improper healing of the skin. Pendulous wing-like folds of skin form on the cat's back, shoulders and haunches. Even stroking the cat can cause the skin to stretch and tear. A recessive autosomal form of feline cutaneous asthenia has been identified in Siamese cats and related breeds. In the homozygous state, it is apparently lethal. Feline cutaneous asthenia is similar to the Ehlers–Danlos syndrome of humans.












