
Fluorocarbons are chemical compounds with carbon-fluorine bonds. Compounds that contain many C-F bonds often have distinctive properties, e.g., enhanced stability, volatility, and hydrophobicity. Several fluorocarbons and their derivatives are commercial polymers, refrigerants, drugs, and anesthetics.

Manganese(III) fluoride (also known as Manganese trifluoride) is the inorganic compound with the formula MnF3. This red/purplish solid is useful for converting hydrocarbons into fluorocarbons, i.e., it is a fluorination agent. It forms a hydrate and many derivatives.

Nickel(II) fluoride is the chemical compound with the formula NiF2. It is an ionic compound of nickel and fluorine and forms yellowish to green tetragonal crystals. Unlike many fluorides, NiF2 is stable in air.

Bromine pentafluoride, BrF5, is an interhalogen compound and a fluoride of bromine. It is a strong fluorinating agent.
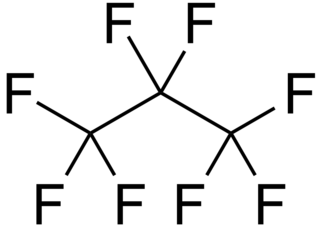
Octafluoropropane (C3F8) is the perfluorocarbon counterpart to the hydrocarbon propane. This non-flammable and non-toxic synthetic substance has applications in semiconductor production and medicine. It is also an extremely potent greenhouse gas.
Tetrafluoroethylene (TFE) is a fluorocarbon with the chemical formula C2F4. It is the simplest perfluorinated alkene. This gaseous species is used primarily in the industrial preparation of fluoropolymers.

Cobalt(III) fluoride is the inorganic compound with the formula CoF3. Hydrates are also known. The anhydrous compound is a hygroscopic brown solid. It is used to synthesize organofluorine compounds.
Organofluorine chemistry describes the chemistry of organofluorine compounds, organic compounds that contain a carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil and water repellents to pharmaceuticals, refrigerants, and reagents in catalysis. In addition to these applications, some organofluorine compounds are pollutants because of their contributions to ozone depletion, global warming, bioaccumulation, and toxicity. The area of organofluorine chemistry often requires special techniques associated with the handling of fluorinating agents.
Perfluorodecalin is a fluorocarbon, a derivative of decalin in which all of the hydrogen atoms are replaced by fluorine atoms. It is chemically and biologically inert and stable up to 400 °C. Several applications make use of its ability to dissolve gases.

Fluorine is a chemical element; it has symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. Fluorine is extremely reactive, as it reacts with all other elements except for the light inert gases.

Perfluorooctane, also known as octadecafluorooctane, is a fluorocarbon liquid—a perfluorinated derivative of the hydrocarbon octane. It can be a good substitute for insulating oil in high voltage electronics. In addition to heat transfer applications, it has also been used as a breathable fluid in partial liquid ventilation.
A hexafluoride is a chemical compound with the general formula QXnF6, QXnF6m−, or QXnF6m+. Many molecules fit this formula. An important hexafluoride is hexafluorosilicic acid (H2SiF6), which is a byproduct of the mining of phosphate rock. In the nuclear industry, uranium hexafluoride (UF6) is an important intermediate in the purification of this element.
Electrochemical fluorination (ECF), or electrofluorination, is a foundational organofluorine chemistry method for the preparation of fluorocarbon-based organofluorine compounds. The general approach represents an application of electrosynthesis. The fluorinated chemical compounds produced by ECF are useful because of their distinctive solvation properties and the relative inertness of carbon–fluorine bonds. Two ECF synthesis routes are commercialized and commonly applied: the Simons process and the Phillips Petroleum process. It is also possible to electrofluorinate in various organic media. Prior to the development of these methods, fluorination with fluorine, a dangerous oxidizing agent, was a dangerous and wasteful process. ECF can be cost-effective, but it may also result in low yields.

Neptunium(VI) fluoride (NpF6) is the highest fluoride of neptunium, it is also one of seventeen known binary hexafluorides. It is an orange volatile crystalline solid. It is relatively hard to handle, being very corrosive, volatile and radioactive. Neptunium hexafluoride is stable in dry air but reacts vigorously with water.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.
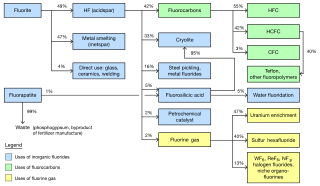
The global market for chemicals from fluorine was about US$16 billion per year as of 2006. The industry was predicted to reach 2.6 million metric tons per year by 2015. The largest market is the United States. Western Europe is the second largest. Asia Pacific is the fastest growing region of production. China in particular has experienced significant growth as a fluorochemical market and is becoming a producer of them as well. Fluorite mining was estimated in 2003 to be a $550 million industry, extracting 4.5 million tons per year.

Perfluoro-1,3-dimethylcyclohexane is a fluorocarbon liquid—a perfluorinated derivative of the hydrocarbon 1,3-dimethylcyclohexane. It is chemically and biologically inert.

Perfluoromethylcyclohexane is a fluorocarbon liquid—a perfluorinated derivative of the hydrocarbon methylcyclohexane. It is chemically and biologically inert.
Perfluoromethyldecalin is a fluorocarbon liquid—a perfluorinated derivative of the hydrocarbon methyldecalin. It is chemically and biologically inert. It is mainly of interest as a blood substitute, exploiting the high solubility of air in this solvent.












