Developmental biology is the study of the process by which animals and plants grow and develop. Developmental biology also encompasses the biology of regeneration, asexual reproduction, metamorphosis, and the growth and differentiation of stem cells in the adult organism.
Morphogenesis is the biological process that causes a cell, tissue or organism to develop its shape. It is one of three fundamental aspects of developmental biology along with the control of tissue growth and patterning of cellular differentiation.

In the developing chordate, the neural tube is the embryonic precursor to the central nervous system, which is made up of the brain and spinal cord. The neural groove gradually deepens as the neural folds become elevated, and ultimately the folds meet and coalesce in the middle line and convert the groove into the closed neural tube. In humans, neural tube closure usually occurs by the fourth week of pregnancy.

Sonic hedgehog protein (SHH) is encoded for by the SHH gene. The protein is named after the video game character Sonic the Hedgehog.

Drosophila embryogenesis, the process by which Drosophila embryos form, is a favorite model system for genetics and developmental biology. The study of its embryogenesis unlocked the century-long puzzle of how development was controlled, creating the field of evolutionary developmental biology. The small size, short generation time, and large brood size make it ideal for genetic studies. Transparent embryos facilitate developmental studies. Drosophila melanogaster was introduced into the field of genetic experiments by Thomas Hunt Morgan in 1909.

Embryoid bodies (EBs) are three-dimensional aggregates formed by pluripotent stem cells. These include embryonic stem cells (ESC) and induced pluripotent stem cells (iPSC)

A morphogen is a substance whose non-uniform distribution governs the pattern of tissue development in the process of morphogenesis or pattern formation, one of the core processes of developmental biology, establishing positions of the various specialized cell types within a tissue. More specifically, a morphogen is a signaling molecule that acts directly on cells to produce specific cellular responses depending on its local concentration.

Lewis Wolpert was a South African-born British developmental biologist, author, and broadcaster. Wolpert popularized his French flag model of embryonic development, using the colours of the French flag as a visual aid to explain how embryonic cells interpret genetic code for expressing characteristics of living organisms and explaining how signalling between cells early in morphogenesis could inform cells with the same genetic regulatory network of their position and role.

The science of pattern formation deals with the visible, (statistically) orderly outcomes of self-organization and the common principles behind similar patterns in nature.
Compartments can be simply defined as separate, different, adjacent cell populations, which upon juxtaposition, create a lineage boundary. This boundary prevents cell movement from cells from different lineages across this barrier, restricting them to their compartment. Subdivisions are established by morphogen gradients and maintained by local cell-cell interactions, providing functional units with domains of different regulatory genes, which give rise to distinct fates. Compartment boundaries are found across species. In the hindbrain of vertebrate embryos, rhombomeres are compartments of common lineage outlined by expression of Hox genes. In invertebrates, the wing imaginal disc of Drosophila provides an excellent model for the study of compartments. Although other tissues, such as the abdomen, and even other imaginal discs are compartmentalized, much of our understanding of key concepts and molecular mechanisms involved in compartment boundaries has been derived from experimentation in the wing disc of the fruit fly.

Ultrabithorax (Ubx) is a homeobox gene found in insects, and is used in the regulation of patterning in morphogenesis. There are many possible products of this gene, which function as transcription factors. Ubx is used in the specification of serially homologous structures, and is used at many levels of developmental hierarchies. In Drosophila melanogaster it is expressed in the third thoracic (T3) and first abdominal (A1) segments and represses wing formation. The Ubx gene regulates the decisions regarding the number of wings and legs the adult flies will have. The developmental role of the Ubx gene is determined by the splicing of its product, which takes place after translation of the gene. The specific splice factors of a particular cell allow the specific regulation of the developmental fate of that cell, by making different splice variants of transcription factors. In D. melanogaster, at least six different isoforms of Ubx exist.
Decapentaplegic (Dpp) is a key morphogen involved in the development of the fruit fly Drosophila melanogaster and is the first validated secreted morphogen. It is known to be necessary for the correct patterning and development of the early Drosophila embryo and the fifteen imaginal discs, which are tissues that will become limbs and other organs and structures in the adult fly. It has also been suggested that Dpp plays a role in regulating the growth and size of tissues. Flies with mutations in decapentaplegic fail to form these structures correctly, hence the name. Dpp is the Drosophila homolog of the vertebrate bone morphogenetic proteins (BMPs), which are members of the TGF-β superfamily, a class of proteins that are often associated with their own specific signaling pathway. Studies of Dpp in Drosophila have led to greater understanding of the function and importance of their homologs in vertebrates like humans.
Limb development in vertebrates is an area of active research in both developmental and evolutionary biology, with much of the latter work focused on the transition from fin to limb.
Within the field of developmental biology, one goal is to understand how a particular cell develops into a final cell type, known as fate determination. Within an embryo, several processes play out at the cellular and tissue level to create an organism. These processes include cell proliferation, differentiation, cellular movement and programmed cell death. Each cell in an embryo receives molecular signals from neighboring cells in the form of proteins, RNAs and even surface interactions. Almost all animals undergo a similar sequence of events during very early development, a conserved process known as embryogenesis. During embryogenesis, cells exist in three germ layers, and undergo gastrulation. While embryogenesis has been studied for more than a century, it was only recently that scientists discovered that a basic set of the same proteins and mRNAs are involved in embryogenesis. Evolutionary conservation is one of the reasons that model systems such as the fly, the mouse, and other organisms are used as models to study embryogenesis and developmental biology. Studying model organisms provides information relevant to other animals, including humans. While studying the different model systems, cells fate was discovered to be determined via multiple ways, two of which are by the combination of transcription factors the cells have and by the cell-cell interaction. Cells' fate determination mechanisms were categorized into three different types, autonomously specified cells, conditionally specified cells, or syncytial specified cells. Furthermore, the cells' fate was determined mainly using two types of experiments, cell ablation and transplantation. The results obtained from these experiments, helped in identifying the fate of the examined cells.
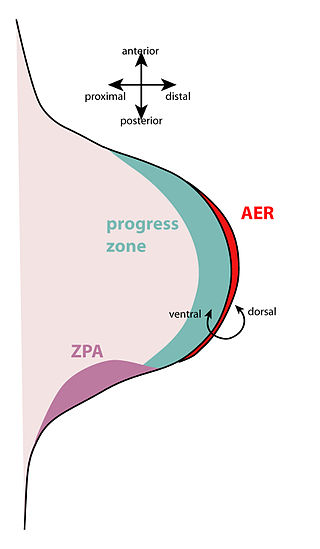
The zone of polarizing activity (ZPA) is an area of mesenchyme that contains signals which instruct the developing limb bud to form along the anterior/posterior axis. Limb bud is undifferentiated mesenchyme enclosed by an ectoderm covering. Eventually, the limb bud develops into bones, tendons, muscles and joints. Limb bud development relies not only on the ZPA, but also many different genes, signals, and a unique region of ectoderm called the apical ectodermal ridge (AER). Research by Saunders and Gasseling in 1948 identified the AER and its subsequent involvement in proximal distal outgrowth. Twenty years later, the same group did transplantation studies in chick limb bud and identified the ZPA. It wasn't until 1993 that Todt and Fallon showed that the AER and ZPA are dependent on each other.

Cytonemes are thin, cellular projections that are specialized for exchange of signaling proteins between cells. Cytonemes emanate from cells that make signaling proteins, extending directly to cells that receive signaling proteins. Cytonemes also extend directly from cells that receive signaling proteins to cells that make them.

The Turing pattern is a concept introduced by English mathematician Alan Turing in a 1952 paper titled "The Chemical Basis of Morphogenesis" which describes how patterns in nature, such as stripes and spots, can arise naturally and autonomously from a homogeneous, uniform state. The pattern arises due to Turing instability which in turn arises due to the interplay between differential diffusion of chemical species and chemical reaction. The instability mechanism is unforeseen because a pure diffusion process would be anticipated to have a stabilizing influence on the system.
Dally is the name of a gene that encodes a HS-modified-protein found in the fruit fly. The protein has to be processed after being codified, and in its mature form it is composed by 626 amino acids, forming a proteoglycan rich in heparin sulfate which is anchored to the cell surface via covalent linkage to glycophosphatidylinositol (GPI), so we can define it as a glypican. For its normal biosynthesis it requires sugarless (sgl), a gene that encodes an enzyme which plays a critical role in the process of modification of dally.
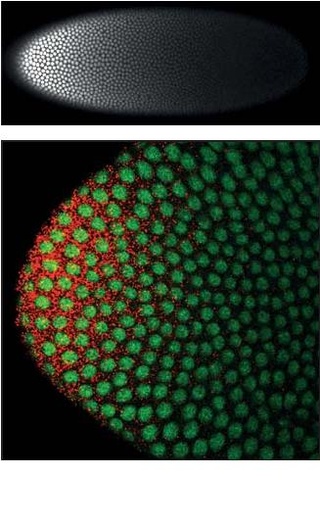
Homeotic protein bicoid is encoded by the bcd maternal effect gene in Drosophilia. Homeotic protein bicoid concentration gradient patterns the anterior-posterior (A-P) axis during Drosophila embryogenesis. Bicoid was the first protein demonstrated to act as a morphogen. Although bicoid is important for the development of Drosophila and other higher dipterans, it is absent from most other insects, where its role is accomplished by other genes.
Thomas Lecuit, born 4 October 1971 in Saumur, is a French biologist specializing in the emergence of forms or morphogenesis. He is a professor at the Collège de France, holding the Dynamics of Life Chair. He leads a research team at the Institut de Biologie du Développement de Marseille (IBDM), and the Turing Centre for Living Systems, an interdisciplinary centre dedicated to the study of living organisms.














