Related Research Articles
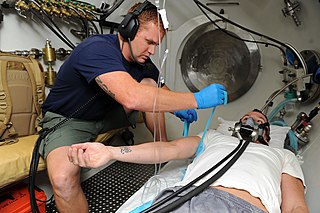
Decompression sickness is a medical condition caused by dissolved gases emerging from solution as bubbles inside the body tissues during decompression. DCS most commonly occurs during or soon after a decompression ascent from underwater diving, but can also result from other causes of depressurisation, such as emerging from a caisson, decompression from saturation, flying in an unpressurised aircraft at high altitude, and extravehicular activity from spacecraft. DCS and arterial gas embolism are collectively referred to as decompression illness.
An air embolism, also known as a gas embolism, is a blood vessel blockage caused by one or more bubbles of air or other gas in the circulatory system. Air can be introduced into the circulation during surgical procedures, lung over-expansion injury, decompression, and a few other causes. In flora, air embolisms may also occur in the xylem of vascular plants, especially when suffering from water stress.
Diving physics, or the physics of underwater diving is the basic aspects of physics which describe the effects of the underwater environment on the underwater diver and their equipment, and the effects of blending, compressing, and storing breathing gas mixtures, and supplying them for use at ambient pressure. These effects are mostly consequences of immersion in water, the hydrostatic pressure of depth and the effects of pressure and temperature on breathing gases. An understanding of the physics behind is useful when considering the physiological effects of diving, breathing gas planning and management, diver buoyancy control and trim, and the hazards and risks of diving.
Decompression Illness (DCI) comprises two different conditions caused by rapid decompression of the body. These conditions present similar symptoms and require the same initial first aid. Scuba divers are trained to ascend slowly from depth to avoid DCI. Although the incidence is relatively rare, the consequences can be serious and potentially fatal, especially if untreated.
Dysbarism refers to medical conditions resulting from changes in ambient pressure. Various activities are associated with pressure changes. Underwater diving is the most frequently cited example, but pressure changes also affect people who work in other pressurized environments, and people who move between different altitudes.
Diving disorders, or diving related medical conditions, are conditions associated with underwater diving, and include both conditions unique to underwater diving, and those that also occur during other activities. This second group further divides into conditions caused by exposure to ambient pressures significantly different from surface atmospheric pressure, and a range of conditions caused by general environment and equipment associated with diving activities.
In physiology, isobaric counterdiffusion (ICD) is the diffusion of different gases into and out of tissues while under a constant ambient pressure, after a change of gas composition, and the physiological effects of this phenomenon. The term inert gas counterdiffusion is sometimes used as a synonym, but can also be applied to situations where the ambient pressure changes. It has relevance in mixed gas diving and anesthesiology.

The decompression of a diver is the reduction in ambient pressure experienced during ascent from depth. It is also the process of elimination of dissolved inert gases from the diver's body which accumulate during ascent, largely during pauses in the ascent known as decompression stops, and after surfacing, until the gas concentrations reach equilibrium. Divers breathing gas at ambient pressure need to ascend at a rate determined by their exposure to pressure and the breathing gas in use. A diver who only breathes gas at atmospheric pressure when free-diving or snorkelling will not usually need to decompress. Divers using an atmospheric diving suit do not need to decompress as they are never exposed to high ambient pressure.
Hypobaric decompression is the reduction in ambient pressure below the normal range of sea level atmospheric pressure. Altitude decompression is hypobaric decompression which is the natural consequence of unprotected elevation to altitude, while other forms of hypobaric decompression are due to intentional or unintentional release of pressurization of a pressure suit or pressurized compartment, vehicle or habitat, and may be controlled or uncontrolled, or the reduction of pressure in a hypobaric chamber.

To prevent or minimize decompression sickness, divers must properly plan and monitor decompression. Divers follow a decompression model to safely allow the release of excess inert gases dissolved in their body tissues, which accommodated as a result of breathing at ambient pressures greater than surface atmospheric pressure. Decompression models take into account variables such as depth and time of dive, breathing gasses, altitude, and equipment to develop appropriate procedures for safe ascent.

Decompression in the context of diving derives from the reduction in ambient pressure experienced by the diver during the ascent at the end of a dive or hyperbaric exposure and refers to both the reduction in pressure and the process of allowing dissolved inert gases to be eliminated from the tissues during this reduction in pressure.

Decompression theory is the study and modelling of the transfer of the inert gas component of breathing gases from the gas in the lungs to the tissues and back during exposure to variations in ambient pressure. In the case of underwater diving and compressed air work, this mostly involves ambient pressures greater than the local surface pressure, but astronauts, high altitude mountaineers, and travellers in aircraft which are not pressurised to sea level pressure, are generally exposed to ambient pressures less than standard sea level atmospheric pressure. In all cases, the symptoms caused by decompression occur during or within a relatively short period of hours, or occasionally days, after a significant pressure reduction.
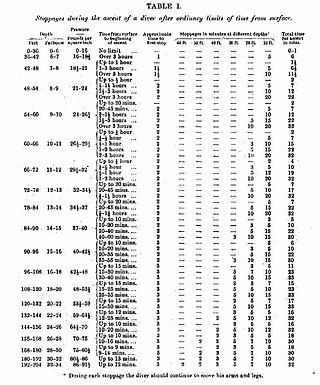
Haldane's decompression model is a mathematical model for decompression to sea level atmospheric pressure of divers breathing compressed air at ambient pressure that was proposed in 1908 by the Scottish physiologist, John Scott Haldane, who was also famous for intrepid self-experimentation.

The thermodynamic model was one of the first decompression models in which decompression is controlled by the volume of gas bubbles coming out of solution. In this model, pain only DCS is modelled by a single tissue which is diffusion-limited for gas uptake and bubble-formation during decompression causes "phase equilibration" of partial pressures between dissolved and free gases. The driving mechanism for gas elimination in this tissue is inherent unsaturation, also called partial pressure vacancy or the oxygen window, where oxygen metabolised is replaced by more soluble carbon dioxide. This model was used to explain the effectiveness of the Torres Straits Island pearl divers empirically developed decompression schedules, which used deeper decompression stops and less overall decompression time than the current naval decompression schedules. This trend to deeper decompression stops has become a feature of more recent decompression models.

The physiology of decompression is the aspect of physiology which is affected by exposure to large changes in ambient pressure. It involves a complex interaction of gas solubility, partial pressures and concentration gradients, diffusion, bulk transport and bubble mechanics in living tissues. Gas is breathed at ambient pressure, and some of this gas dissolves into the blood and other fluids. Inert gas continues to be taken up until the gas dissolved in the tissues is in a state of equilibrium with the gas in the lungs, or the ambient pressure is reduced until the inert gases dissolved in the tissues are at a higher concentration than the equilibrium state, and start diffusing out again.
Diving hazards are the agents or situations that pose a threat to the underwater diver or their equipment. Divers operate in an environment for which the human body is not well suited. They face special physical and health risks when they go underwater or use high pressure breathing gas. The consequences of diving incidents range from merely annoying to rapidly fatal, and the result often depends on the equipment, skill, response and fitness of the diver and diving team. The classes of hazards include the aquatic environment, the use of breathing equipment in an underwater environment, exposure to a pressurised environment and pressure changes, particularly pressure changes during descent and ascent, and breathing gases at high ambient pressure. Diving equipment other than breathing apparatus is usually reliable, but has been known to fail, and loss of buoyancy control or thermal protection can be a major burden which may lead to more serious problems. There are also hazards of the specific diving environment, and hazards related to access to and egress from the water, which vary from place to place, and may also vary with time. Hazards inherent in the diver include pre-existing physiological and psychological conditions and the personal behaviour and competence of the individual. For those pursuing other activities while diving, there are additional hazards of task loading, of the dive task and of special equipment associated with the task.

A built-in breathing system is a source of breathing gas installed in a confined space where an alternative to the ambient gas may be required for medical treatment, emergency use, or to minimise a hazard. They are found in diving chambers, hyperbaric treatment chambers, and submarines.
Inner ear decompression sickness, (IEDCS) or audiovestibular decompression sickness is a medical condition of the inner ear caused by the formation of gas bubbles in the tissues or blood vessels of the inner ear. Generally referred to as a form of decompression sickness, it can also occur at constant pressure due to inert gas counterdiffusion effects.
References
- ↑ Bohl, M (May 1997). "Gas bubble disease of fish". Tierarztliche Praxis. 25 (3): 284–288. PMID 9289892.
- ↑ Gültepe, N; Ateş, O; Hisar, O (Sep 2011). "Carbonic anhydrase activities from the rainbow trout lens correspond to the development of acute gas bubble disease". Journal of Aquatic Animal Health. 23 (3): 134–139. Bibcode:2011JAqAH..23..134G. doi:10.1080/08997659.2011.616848. PMID 22216712.
- 1 2 3 4 5 6 7 Rucker, Robert R. (1972). Gas-bubble Disease of Salmonids: A Critical Review. Bureau of Sport Fisheries and Wildlife.
- ↑ Hulst, Robert A. van; Klein, Jan; Lachmann, Burkhard (2003). "Gas embolism: pathophysiology and treatment". Clinical Physiology and Functional Imaging. 23 (5): 237–246. doi:10.1046/j.1475-097X.2003.00505.x. ISSN 1475-097X. PMID 12950319. S2CID 24087721.
- 1 2 3 4 "Fish Diseases". aun.edu.eg. Retrieved 2019-12-08.
- 1 2 3 4 5 Bouck, Gerald R. (1980-11-01). "Etiology of Gas Bubble Disease". Transactions of the American Fisheries Society. 109 (6): 703–707. Bibcode:1980TrAFS.109..703B. doi:10.1577/1548-8659(1980)109<703:eogbd>2.0.co;2. ISSN 0002-8487.