
Metallurgy is a domain of materials science and engineering that studies the physical and chemical behavior of metallic elements, their inter-metallic compounds, and their mixtures, which are known as alloys.

Titanium is a chemical element; it has symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion in sea water, aqua regia, and chlorine.

Chrome plating is a technique of electroplating a thin layer of chromium onto a metal object. A chrome plated part is called chrome, or is said to have been chromed. The chromium layer can be decorative, provide corrosion resistance, facilitate cleaning, and increase surface hardness. Sometimes a less expensive substitute for chrome, such as nickel, may be used for aesthetic purposes.
Refractory metals are a class of metals that are extraordinarily resistant to heat and wear. The expression is mostly used in the context of materials science, metallurgy and engineering. The definition of which elements belong to this group differs. The most common definition includes five elements: two of the fifth period and three of the sixth period. They all share some properties, including a melting point above 2000 °C and high hardness at room temperature. They are chemically inert and have a relatively high density. Their high melting points make powder metallurgy the method of choice for fabricating components from these metals. Some of their applications include tools to work metals at high temperatures, wire filaments, casting molds, and chemical reaction vessels in corrosive environments. Partly due to the high melting point, refractory metals are stable against creep deformation to very high temperatures.

Anodizing is an electrolytic passivation process used to increase the thickness of the natural oxide layer on the surface of metal parts.

Hydrogen embrittlement (HE), also known as hydrogen-assisted cracking or hydrogen-induced cracking (HIC), is a reduction in the ductility of a metal due to absorbed hydrogen. Hydrogen atoms are small and can permeate solid metals. Once absorbed, hydrogen lowers the stress required for cracks in the metal to initiate and propagate, resulting in embrittlement. Hydrogen embrittlement occurs in steels, as well as in iron, nickel, titanium, cobalt, and their alloys. Copper, aluminium, and stainless steels are less susceptible to hydrogen embrittlement.
Plating is a finishing process in which a metal is deposited on a surface. Plating has been done for hundreds of years; it is also critical for modern technology. Plating is used to decorate objects, for corrosion inhibition, to improve solderability, to harden, to improve wearability, to reduce friction, to improve paint adhesion, to alter conductivity, to improve IR reflectivity, for radiation shielding, and for other purposes. Jewelry typically uses plating to give a silver or gold finish.

Galling is a form of wear caused by adhesion between sliding surfaces. When a material galls, some of it is pulled with the contacting surface, especially if there is a large amount of force compressing the surfaces together. Galling is caused by a combination of friction and adhesion between the surfaces, followed by slipping and tearing of crystal structure beneath the surface. This will generally leave some material stuck or even friction welded to the adjacent surface, whereas the galled material may appear gouged with balled-up or torn lumps of material stuck to its surface.

Diamond-like carbon (DLC) is a class of amorphous carbon material that displays some of the typical properties of diamond. DLC is usually applied as coatings to other materials that could benefit from such properties.

Titanium nitride is an extremely hard ceramic material, often used as a physical vapor deposition (PVD) coating on titanium alloys, steel, carbide, and aluminium components to improve the substrate's surface properties.
Fretting refers to wear and sometimes corrosion damage of loaded surfaces in contact while they encounter small oscillatory movements tangential to the surface. Fretting is caused by adhesion of contact surface asperities, which are subsequently broken again by the small movement. This breaking causes wear debris to be formed.

Chromium(II) carbide is a ceramic compound that exists in several chemical compositions: Cr3C2, Cr7C3, and Cr23C6. At standard conditions it exists as a gray solid. It is extremely hard and corrosion resistant. It is also a refractory compound, which means that it retains its strength at high temperatures as well. These properties make it useful as an additive to metal alloys. When chromium carbide crystals are integrated into the surface of a metal it improves the wear resistance and corrosion resistance of the metal, and maintains these properties at elevated temperatures. The hardest and most commonly used composition for this purpose is Cr3C2.

Plasma electrolytic oxidation (PEO), also known as electrolytic plasma oxidation (EPO) or microarc oxidation (MAO), is an electrochemical surface treatment process for generating oxide coatings on metals. It is similar to anodizing, but it employs higher potentials, so that discharges occur and the resulting plasma modifies the structure of the oxide layer. This process can be used to grow thick, largely crystalline, oxide coatings on metals such as aluminium, magnesium and titanium. Because they can present high hardness and a continuous barrier, these coatings can offer protection against wear, corrosion or heat as well as electrical insulation.

Electroless nickel-phosphorus plating, also referred to as E-nickel, is a chemical process that deposits an even layer of nickel-phosphorus alloy on the surface of a solid substrate, like metal or plastic. The process involves dipping the substrate in a water solution containing nickel salt and a phosphorus-containing reducing agent, usually a hypophosphite salt. It is the most common version of electroless nickel plating and is often referred by that name. A similar process uses a borohydride reducing agent, yielding a nickel-boron coating instead.
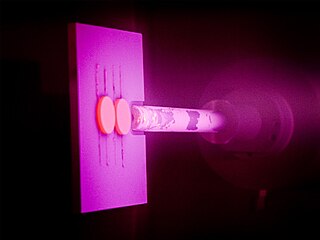
Physical vapor deposition (PVD), sometimes called physical vapor transport (PVT), describes a variety of vacuum deposition methods which can be used to produce thin films and coatings on substrates including metals, ceramics, glass, and polymers. PVD is characterized by a process in which the material transitions from a condensed phase to a vapor phase and then back to a thin film condensed phase. The most common PVD processes are sputtering and evaporation. PVD is used in the manufacturing of items which require thin films for optical, mechanical, electrical, acoustic or chemical functions. Examples include semiconductor devices such as thin-film solar cells, microelectromechanical devices such as thin film bulk acoustic resonator, aluminized PET film for food packaging and balloons, and titanium nitride coated cutting tools for metalworking. Besides PVD tools for fabrication, special smaller tools used mainly for scientific purposes have been developed.
Phosphate conversion coating is a chemical treatment applied to steel parts that creates a thin adhering layer of iron, zinc, or manganese phosphates to improve corrosion resistance or lubrication or as a foundation for subsequent coatings or painting. It is one of the most common types of conversion coating. The process is also called phosphate coating, phosphatization, phosphatizing, or phosphating. It is also known by the trade name Parkerizing, especially when applied to firearms and other military equipment.
Anti-scratch coating is a type of protective coating or film applied to an object's surface for mitigation against scratches. Scratches are small surface-level cuts left on a surface following interaction with a sharper object. Anti-scratch coatings provide scratch resistances by containing tiny microscopic materials with scratch-resistant properties. Scratch resistance materials come in the form of additives, filters, and binders. Besides materials, scratch resistances is impacted by coating formation techniques. Scratch resistance is measured using the Scratch-hardness test. Commercially, anti-scratch coatings are used in the automotive, optical, photographic, and electronics industries, where resale and/or functionality is impaired by scratches. Anti-scratch coatings are of growing importance as traditional scratch resistance materials like metals and glass are replaced with low-scratch resistant plastics.
Aluminium magnesium boride or Al3Mg3B56, colloquially known as BAM, is a chemical compound of aluminium, magnesium and boron. Whereas its nominal formula is AlMgB14, the chemical composition is closer to Al0.75Mg0.75B14. It is a ceramic alloy that is highly resistive to wear and has an extremely low coefficient of sliding friction, reaching a record value of 0.04 in unlubricated and 0.02 in lubricated AlMgB14−TiB2 composites. First reported in 1970, BAM has an orthorhombic structure with four icosahedral B12 units per unit cell. This ultrahard material has a coefficient of thermal expansion comparable to that of other widely used materials such as steel and concrete.

Titanium aluminium nitride (TiAlN) or aluminium titanium nitride is a group of metastable hard coatings consisting of nitrogen and the metallic elements aluminium and titanium. This compound as well as similar compounds(such as TiN and TiCN) are most notably used for coating machine tools such and endmills and drills to change their properties, such as increased thermal stability and/or wear resistance. Four important compositions are deposited in industrial scale by physical vapor deposition methods:
Titanium adhesive bonding is an engineering process used in the aerospace industry, medical-device manufacture and elsewhere. Titanium alloy is often used in medical and military applications because of its strength, weight, and corrosion resistance characteristics. In implantable medical devices, titanium is used because of its biocompatibility and its passive, stable oxide layer. Also, titanium allergies are rare and in those cases mitigations like Parylene coating are used. In the aerospace industry titanium is often bonded to save cost, touch times, and the need for mechanical fasteners. In the past, Russian submarines' hulls were completely made of titanium because the non-magnetic nature of the material went undetected by the defense technology at that time. Bonding adhesive to titanium requires preparing the surface beforehand, and there is not a single solution for all applications. For example, etchant and chemical methods are not biocompatible and cannot be employed when the device will come into contact with blood and tissue. Mechanical surface roughness techniques like sanding and laser roughening may make the surface brittle and create micro-hardness regions that would not be suitable for cyclic loading found in military applications. Air oxidation at high temperatures will produce a crystalline oxide layer at a lower investment cost, but the increased temperatures can deform precision parts. The type of adhesive, thermosetting or thermoplastic, and curing methods are also factors in titanium bonding because of the adhesive's interaction with the treated oxide layer. Surface treatments can also be combined. For example, a grit blast process can be followed by a chemical etch and a primer application.













