Related Research Articles

Angiography or arteriography is a medical imaging technique used to visualize the inside, or lumen, of blood vessels and organs of the body, with particular interest in the arteries, veins, and the heart chambers. Modern angiography is performed by injecting a radio-opaque contrast agent into the blood vessel and imaging using X-ray based techniques such as fluoroscopy.

Optical coherence tomography (OCT) is an imaging technique that uses low-coherence light to capture micrometer-resolution, two- and three-dimensional images from within optical scattering media. It is used for medical imaging and industrial nondestructive testing (NDT). Optical coherence tomography is based on low-coherence interferometry, typically employing near-infrared light. The use of relatively long wavelength light allows it to penetrate into the scattering medium. Confocal microscopy, another optical technique, typically penetrates less deeply into the sample but with higher resolution.

Coronary thrombosis is defined as the formation of a blood clot inside a blood vessel of the heart. This blood clot may then restrict blood flow within the heart, leading to heart tissue damage, or a myocardial infarction, also known as a heart attack.
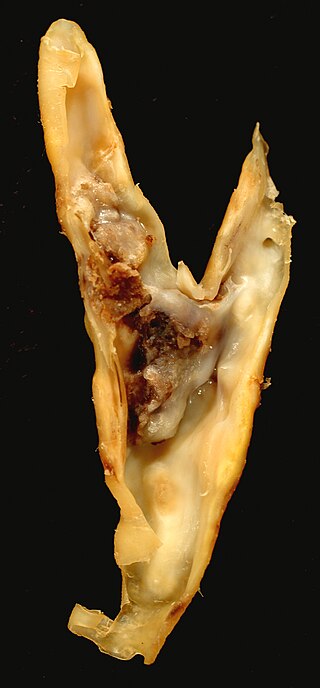
An atheroma, or atheromatous plaque, is an abnormal accumulation of material in the inner layer of an arterial wall.
Intravascular ultrasound (IVUS) or intravascular echocardiography is a medical imaging methodology using a specially designed catheter with a miniaturized ultrasound probe attached to the distal end of the catheter. The proximal end of the catheter is attached to computerized ultrasound equipment. It allows the application of ultrasound technology, such as piezoelectric transducer or CMUT, to see from inside blood vessels out through the surrounding blood column, visualizing the endothelium of blood vessels.

Percutaneous coronary intervention (PCI) is a non-surgical procedure used to treat narrowing of the coronary arteries of the heart found in coronary artery disease. The process involves combining coronary angioplasty with stenting, which is the insertion of a permanent wire-meshed tube that is either drug eluting (DES) or composed of bare metal (BMS). The stent delivery balloon from the angioplasty catheter is inflated with media to force contact between the struts of the stent and the vessel wall, thus widening the blood vessel diameter. After accessing the blood stream through the femoral or radial artery, the procedure uses coronary catheterization to visualise the blood vessels on X-ray imaging. After this, an interventional cardiologist can perform a coronary angioplasty, using a balloon catheter in which a deflated balloon is advanced into the obstructed artery and inflated to relieve the narrowing; certain devices such as stents can be deployed to keep the blood vessel open. Various other procedures can also be performed.
The history of invasive and interventional cardiology is complex, with multiple groups working independently on similar technologies. Invasive and interventional cardiology is currently closely associated with cardiologists, though the development and most of its early research and procedures were performed by diagnostic and interventional radiologists.
Fractional flow reserve (FFR) is a diagnostic technique used in coronary catheterization. FFR measures pressure differences across a coronary artery stenosis to determine the likelihood that the stenosis impedes oxygen delivery to the heart muscle.

Intima–media thickness (IMT), also called intimal medial thickness, is a measurement of the thickness of tunica intima and tunica media, the innermost two layers of the wall of an artery. The measurement is usually made by external ultrasound and occasionally by internal, invasive ultrasound catheters. Measurements of the total wall thickness of blood vessels can also be done using other imaging modalities.
A coronary CT calcium scan is a computed tomography (CT) scan of the heart for the assessment of severity of coronary artery disease. Specifically, it looks for calcium deposits in atherosclerotic plaques in the coronary arteries that can narrow arteries and increase the risk of heart attack. These plaques are the cause of most heart attacks, and become calcified as they mature. These calcifications can then be detected by CT because of their high attenuation. This severity can be presented as an Agatston score or coronary artery calcium (CAC) score. The CAC score is an independent marker of risk for cardiac events, cardiac mortality, and all-cause mortality. In addition, it provides additional prognostic information to other cardiovascular risk markers. Obstructions may be present even with an Agatston score of zero, especially in younger patients. A typical coronary CT calcium scan is done without the use of radiocontrast, but it can possibly be done from contrast-enhanced images as well, such as in coronary CT angiography. The exam is best performed with cardiac gating to eliminate motion but can also be estimated in the presence of motion.

Coronary artery aneurysm is an abnormal dilatation of part of the coronary artery. This rare disorder occurs in about 0.3–4.9% of patients who undergo coronary angiography.

Atherectomy is a minimally invasive technique for removing atherosclerosis from blood vessels within the body. It is an alternative to angioplasty for the treatment of peripheral artery disease, but the studies that exist are not adequate to determine whether it is superior to angioplasty. It has also been used to treat coronary artery disease, albeit without evidence of superiority to angioplasty.

Spontaneous coronary artery dissection (SCAD) is an uncommon but potentially lethal condition in which one of the coronary arteries that supply the heart, spontaneously develops a blood collection, or hematoma, within the artery wall due to a tear in the wall. SCAD is one of the arterial dissections that can occur.

Coronary CT angiography is the use of computed tomography (CT) angiography to assess the coronary arteries of the heart. The patient receives an intravenous injection of radiocontrast and then the heart is scanned using a high speed CT scanner, allowing physicians to assess the extent of occlusion in the coronary arteries, usually in order to diagnose coronary artery disease.
Endomicroscopy is a technique for obtaining histology-like images from inside the human body in real-time, a process known as ‘optical biopsy’. It generally refers to fluorescence confocal microscopy, although multi-photon microscopy and optical coherence tomography have also been adapted for endoscopic use. Commercially available clinical and pre-clinical endomicroscopes can achieve a resolution on the order of a micrometre, have a field-of-view of several hundred µm, and are compatible with fluorophores which are excitable using 488 nm laser light. The main clinical applications are currently in imaging of the tumour margins of the brain and gastro-intestinal tract, particularly for the diagnosis and characterisation of Barrett’s Esophagus, pancreatic cysts and colorectal lesions. A number of pre-clinical and transnational applications have been developed for endomicroscopy as it enables researchers to perform live animal imaging. Major pre-clinical applications are in gastro-intestinal tract, toumour margin detection, uterine complications, ischaemia, live imaging of cartilage and tendon and organoid imaging.
Guillermo J. Tearney is a professor of pathology at Harvard Medical School, a physicist in the department of dermatology at the Massachusetts General Hospital, a pathologist in the department of pathology at the Massachusetts General Hospital and runs a research laboratory at the Wellman Center for Photomedicine at the Massachusetts General Hospital in Boston Massachusetts. Tearney received his BA in applied mathematics, graduating cum laude (1988), his MD graduating magna cum laude (1998) from Harvard Medical School, and received his PhD in electrical engineering (1997) from the Massachusetts Institute of Technology. He is a well-known name in the field of biomedical optics, gastroenterology, and interventional cardiology for his prominent role on the development of endoscopic optical coherence tomography, in particular intracoronary optical coherence tomography, its translation to the clinic, and commercialization. He is recognized as one of the inventors of Intracoronary optical coherence tomography. He is also recognized as co-inventor of optical coherence tomography for endoscopic imaging and diagnosis of esophagus disorders, a clinical technology currently commercialized by NinePoint Medical.

Intracoronary optical coherence tomography (OCT) is an endoscopic-based application of optical coherence tomography. Analogous to intravascular ultrasound, intracoronary OCT uses a catheter to deliver and collect near infrared light to create cross-sectional images of the artery lumen and wall. Intracoronary OCT creates images at a resolution of approximately 15 micro-meters, an order of magnitude improved resolution with respect to intravascular ultrasound and X-ray coronary angiogram.
Intravascular imaging is a catheter based system that allows physicians such as interventional cardiologists to acquire images of diseased vessels from inside the artery. Intravascular imaging provides detailed and accurate measurements of vessel lumen morphology, vessel size, extension of diseased artery segments, vessel size and plaque characteristics. Examples of intravascular imaging modalities are intravascular ultrasound (IVUS) and intracoronary optical coherence tomography.

Intravascular fluorescence is a catheter-based molecular imaging technique that uses near-infrared fluorescence to detect artery wall autofluorescence (NIRAF) or fluorescence generated by molecular agents injected intravenously (NIRF). No commercial systems based on intravascular fluorescence are currently on the market, however, significant steps forwards in intravascular fluorescence imaging technology have been made between 2010-2016. It is typically used to detect functional state of artery wall including some known high-risk features of atherosclerosis. It is usually combined with structural imaging modalities such as Intravascular ultrasound and/or Intracoronary optical coherence tomography, to provide functional information in a morphological context.
Optical coherence elastography (OCE) is an emerging imaging technique used in biomedical imaging to form pictures of biological tissue in micron and submicron level and maps the biomechanical property of tissue.
References
- ↑ "2014 Bullock-Wellman Fellowship Award Recipients". Wellman Center for Photomedicine. Retrieved 8 September 2016.
- ↑ Neale, Todd (11 March 2016). "Does New Dual-Modality Imaging Come Closer to Uncovering Vulnerable Plaques?". TCTMD. Cardiovascular Research Foundation. Retrieved 13 September 2016.
- ↑ Psaltis PJ, Nicholls SJ (2016). "Imaging: Focusing light on the vulnerable plaque". Nature Reviews Cardiology. 13 (5): 253–255. doi: 10.1038/nrcardio.2016.53 . PMID 27087409. S2CID 166201.
- ↑ Ughi GJ, Wang H, Gerbaud E, Gardecki JA, Fard AM, Hamidi E, Vacas-Jacques P, Rosenberg M, Jaffer FA, Tearney GJ (2016). "Clinical Characterization of Coronary Atherosclerosis With Dual-Modality OCT and Near-Infrared Autofluorescence Imaging". J Am Coll Cardiol Img. 9 (11): 1304–1314. doi:10.1016/j.jcmg.2015.11.020. PMC 5010789 . PMID 26971006.
- ↑ "Combining two imaging technologies may better identify dangerous coronary plaques – Adding fluorescence imaging to OCT reveals biological, as well as structural information". Mass General News. Retrieved 7 September 2016.
- ↑ "OCT, fluorescence imaging pair to better identify heart attack-prone coronary plaques". 11 March 2016. Retrieved 11 September 2016.
- ↑ Verjans JW, Osborn EA, Ughi GJ, Calfon Press MA, Hamidi E, Antoniadis AP, Papafaklis MI, Conrad MF, Libby P, Stone PH, Cambria RP, Tearney GJ, Jaffer FA (2016). "Targeted Near-Infrared Fluorescence Imaging of Atherosclerosis: Clinical and Intracoronary Evaluation of Indocyanine Green". J Am Coll Cardiol Img. 9 (9): 1087–1095. doi:10.1016/j.jcmg.2016.01.034. PMC 5136528 . PMID 27544892.
- ↑ Osborn EA, Ughi GJ, Verjans JW, Piao Z, Gerbaud E, Albaghdadi M, Khraishah H, Kassab MB, Takx R, Cui J, Mauskapf A, Shen C, Yeh RW, Klimas MT, Tawakol A, Tearney GJ, Jaffer FA (2021). "Intravascular Molecular-Structural Assessment of Arterial Inflammation in Preclinical Atherosclerosis Progression". J Am Coll Cardiol Img. 14 (11): 2265–2267. doi:10.1016/j.jcmg.2021.06.017. PMC 8571057 . PMID 34419392.
- ↑ Hara T, Ughi GJ, McCarthy JR, Erdem SS, Mauskapf A, Lyon SC, Fard AM, Edelman ER, Tearney GJ, Jaffer FA (2015). "Intravascular fibrin molecular imaging improves the detection of unhealed stents assessed by optical coherence tomography in vivo". European Heart Journal. 38 (6): 447–455. doi:10.1093/eurheartj/ehv677. PMC 5837565 . PMID 26685129.
- ↑ Ughi GJ, Van Dyck CJ, Adriaenssens T, Hoymans VY, Sinnaeve P, Timmermans JP, Desmet W, Vrints CJ, D'hooge J (2014). "Automatic assessment of stent neointimal coverage by intravascular optical coherence tomography". Eur Heart J Cardiovasc Imaging. 15 (2): 195–200. doi: 10.1093/ehjci/jet134 . PMID 23884965.
- ↑ Ughi GJ, Adriaenssens T, Desmet W, D'hooge J (2012). "Fully automatic three-dimensional visualization of intravascular optical coherence tomography images". Biomed Opt Express. 3 (12): 3291–3303. doi:10.1364/boe.3.003291. PMC 3521298 . PMID 23243578.
- ↑ Ughi GJ, Adriaenssens T, Onsea K, Kayaert P, Dubois C, Sinnaeve P, Coosemans M, Desmet W, D'hooge J (2012). "Automatic segmentation of in-vivo intra-coronary optical coherence tomography images to assess stent strut apposition and coverage". Int J Cardiovasc Imaging. 28 (2): 229–241. doi:10.1007/s10554-011-9824-3. PMID 21347593. S2CID 21504211.
- ↑ "winner of Optical Coherence Tomography Award". 9 January 2011. Retrieved 11 September 2016.
- ↑ "Giovanni J. Ughi, PhD". Google Scholar. Retrieved 20 April 2017.
- ↑ "Giovanni J. Ughi, PhD". ORCID. Retrieved 22 August 2021.