Related Research Articles
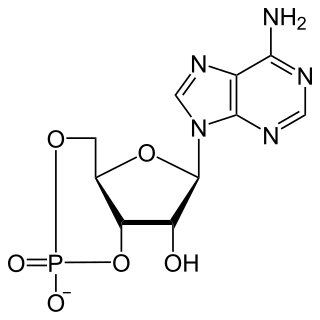
Cyclic adenosine monophosphate is a second messenger, or cellular signal occurring within cells, that is important in many biological processes. cAMP is a derivative of adenosine triphosphate (ATP) and used for intracellular signal transduction in many different organisms, conveying the cAMP-dependent pathway.

G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of evolutionarily related proteins that are cell surface receptors that detect molecules outside the cell and activate cellular responses. They are coupled with G proteins. They pass through the cell membrane seven times in the form of six loops of amino acid residues, which is why they are sometimes referred to as seven-transmembrane receptors. Ligands can bind either to the extracellular N-terminus and loops or to the binding site within transmembrane helices. They are all activated by agonists, although a spontaneous auto-activation of an empty receptor has also been observed.

Signal transduction is the process by which a chemical or physical signal is transmitted through a cell as a series of molecular events. Proteins responsible for detecting stimuli are generally termed receptors, although in some cases the term sensor is used. The changes elicited by ligand binding in a receptor give rise to a biochemical cascade, which is a chain of biochemical events known as a signaling pathway.

In biochemistry, a kinase is an enzyme that catalyzes the transfer of phosphate groups from high-energy, phosphate-donating molecules to specific substrates. This process is known as phosphorylation, where the high-energy ATP molecule donates a phosphate group to the substrate molecule. As a result, kinase produces a phosphorylated substrate and ADP. Conversely, it is referred to as dephosphorylation when the phosphorylated substrate donates a phosphate group and ADP gains a phosphate group. These two processes, phosphorylation and dephosphorylation, occur four times during glycolysis.

Adenosine monophosphate (AMP), also known as 5'-adenylic acid, is a nucleotide. AMP consists of a phosphate group, the sugar ribose, and the nucleobase adenine. It is an ester of phosphoric acid and the nucleoside adenosine. As a substituent it takes the form of the prefix adenylyl-.

A cyclic nucleotide (cNMP) is a single-phosphate nucleotide with a cyclic bond arrangement between the sugar and phosphate groups. Like other nucleotides, cyclic nucleotides are composed of three functional groups: a sugar, a nitrogenous base, and a single phosphate group. As can be seen in the cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) images, the 'cyclic' portion consists of two bonds between the phosphate group and the 3' and 5' hydroxyl groups of the sugar, very often a ribose.

In cell biology, protein kinase A (PKA) is a family of serine-threonine kinase whose activity is dependent on cellular levels of cyclic AMP (cAMP). PKA is also known as cAMP-dependent protein kinase. PKA has several functions in the cell, including regulation of glycogen, sugar, and lipid metabolism. It should not be confused with 5'-AMP-activated protein kinase.

5' AMP-activated protein kinase or AMPK or 5' adenosine monophosphate-activated protein kinase is an enzyme that plays a role in cellular energy homeostasis, largely to activate glucose and fatty acid uptake and oxidation when cellular energy is low. It belongs to a highly conserved eukaryotic protein family and its orthologues are SNF1 in yeast, and SnRK1 in plants. It consists of three proteins (subunits) that together make a functional enzyme, conserved from yeast to humans. It is expressed in a number of tissues, including the liver, brain, and skeletal muscle. In response to binding AMP and ADP, the net effect of AMPK activation is stimulation of hepatic fatty acid oxidation, ketogenesis, stimulation of skeletal muscle fatty acid oxidation and glucose uptake, inhibition of cholesterol synthesis, lipogenesis, and triglyceride synthesis, inhibition of adipocyte lipogenesis, inhibition of adipocyte lipolysis, and modulation of insulin secretion by pancreatic β-cells.

CREB-TF is a cellular transcription factor. It binds to certain DNA sequences called cAMP response elements (CRE), thereby increasing or decreasing the transcription of the genes. CREB was first described in 1987 as a cAMP-responsive transcription factor regulating the somatostatin gene.
Adenylate kinase is a phosphotransferase enzyme that catalyzes the interconversion of the various adenosine phosphates. By constantly monitoring phosphate nucleotide levels inside the cell, ADK plays an important role in cellular energy homeostasis.
A biochemical cascade, also known as a signaling cascade or signaling pathway, is a series of chemical reactions that occur within a biological cell when initiated by a stimulus. This stimulus, known as a first messenger, acts on a receptor that is transduced to the cell interior through second messengers which amplify the signal and transfer it to effector molecules, causing the cell to respond to the initial stimulus. Most biochemical cascades are series of events, in which one event triggers the next, in a linear fashion. At each step of the signaling cascade, various controlling factors are involved to regulate cellular actions, in order to respond effectively to cues about their changing internal and external environments.

Mitogen-activated protein kinase 9 is an enzyme that in humans is encoded by the MAPK9 gene.

5'-AMP-activated protein kinase catalytic subunit alpha-2 is an enzyme that in humans is encoded by the PRKAA2 gene.

5'-AMP-activated protein kinase catalytic subunit alpha-1 is an enzyme that in humans is encoded by the PRKAA1 gene.

5'-AMP-activated protein kinase subunit beta-1 is an enzyme that in humans is encoded by the PRKAB1 gene.

5'-AMP-activated protein kinase subunit gamma-1 is an enzyme that in humans is encoded by the PRKAG1 gene.

5'-AMP-activated protein kinase subunit beta-2 is an enzyme that in humans is encoded by the PRKAB2 gene.

5'-AMP-activated protein kinase subunit gamma-3 is an enzyme that in humans is encoded by the PRKAG3 gene.

Theo Wallimann is a Swiss biologist who was research group leader and Adjunct-Professor at the Institute of Cell Biology ETH Zurich and later at the Institute of Molecular Health Science https://mhs.biol.ethz.ch/about-us/emeriti-formermembers/wallimann.html at the ETH Zurich at the Biology Department https://biol.ethz.ch/en/, of the ETH Zurich, Switzerland.
The Portland Press Excellence in Science Award was an annual award instituted in 1964 to recognize notable research in any branch of biochemistry undertaken in the UK or Republic of Ireland. It was initially called the CIBA Medal and Prize, then the Novartis Medal and Prize. The prize consists of a medal and a £3000 cash award. The winner is invited to present a lecture at a Society conference and submit an article to one of the Society's publications. Notable recipients include the Nobel laureates John E. Walker, Paul Nurse, Sydney Brenner, César Milstein, Peter D. Mitchell, Rodney Porter, and John Cornforth.
References
- ↑ "Professor Grahame Hardie FRS, FRSE, FMedSci". 30 August 2013.
- ↑ "David Grahame Hardie - F1000Prime".
- ↑ Winder WW, Hardie DG (July 1999). "AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes". The American Journal of Physiology. 277 (1): E1-10. doi:10.1152/ajpendo.1999.277.1.E1. PMID 10409121.