Related Research Articles

Boron nitride is a thermally and chemically resistant refractory compound of boron and nitrogen with the chemical formula BN. It exists in various crystalline forms that are isoelectronic to a similarly structured carbon lattice. The hexagonal form corresponding to graphite is the most stable and soft among BN polymorphs, and is therefore used as a lubricant and an additive to cosmetic products. The cubic variety analogous to diamond is called c-BN; it is softer than diamond, but its thermal and chemical stability is superior. The rare wurtzite BN modification is similar to lonsdaleite but slightly softer than the cubic form.

Chemical vapor deposition (CVD) is a vacuum deposition method used to produce high-quality, and high-performance, solid materials. The process is often used in the semiconductor industry to produce thin films.
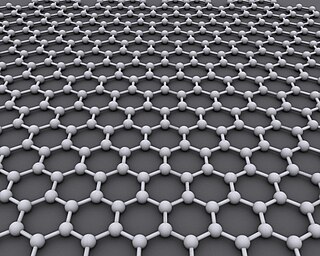
Graphene is an allotrope of carbon consisting of a single layer of atoms arranged in a hexagonal lattice nanostructure. The name is derived from "graphite" and the suffix -ene, reflecting the fact that the graphite allotrope of carbon contains numerous double bonds.
Plating is a finishing process in which a metal is deposited on a surface. Plating has been done for hundreds of years; it is also critical for modern technology. Plating is used to decorate objects, for corrosion inhibition, to improve solderability, to harden, to improve wearability, to reduce friction, to improve paint adhesion, to alter conductivity, to improve IR reflectivity, for radiation shielding, and for other purposes. Jewelry typically uses plating to give a silver or gold finish.

A superalloy, or high-performance alloy, is an alloy with the ability to operate at a high fraction of its melting point. Key characteristics of a superalloy include mechanical strength, thermal creep deformation resistance, surface stability, and corrosion and oxidation resistance.
Fusion bonded epoxy coating, also known as fusion-bond epoxy powder coating and commonly referred to as FBE coating, is an epoxy-based powder coating that is widely used to protect steel pipe used in pipeline construction from corrosion. It is also commonly used to protect reinforcing bars and on a wide variety of piping connections, valves etc. FBE coatings are thermoset polymer coatings. They come under the category of protective coatings in paints and coating nomenclature. The name fusion-bond epoxy is due to resigning cross-link and the application method, which is different from a conventional paint. In 2020 the market size was quoted at 12 billion dollars.

Thermal spraying techniques are coating processes in which melted materials are sprayed onto a surface. The "feedstock" is heated by electrical or chemical means.

Thermal barrier coatings (TBCs) are advanced materials systems usually applied to metallic surfaces on parts operating at elevated temperatures, such as gas turbine combustors and turbines, and in automotive exhaust heat management. These 100 μm to 2 mm thick coatings of thermally insulating materials serve to insulate components from large and prolonged heat loads and can sustain an appreciable temperature difference between the load-bearing alloys and the coating surface. In doing so, these coatings can allow for higher operating temperatures while limiting the thermal exposure of structural components, extending part life by reducing oxidation and thermal fatigue. In conjunction with active film cooling, TBCs permit working fluid temperatures higher than the melting point of the metal airfoil in some turbine applications. Due to increasing demand for more efficient engines running at higher temperatures with better durability/lifetime and thinner coatings to reduce parasitic mass for rotating/moving components, there is significant motivation to develop new and advanced TBCs. The material requirements of TBCs are similar to those of heat shields, although in the latter application emissivity tends to be of greater importance.

A spray nozzle or atomizer is a device that facilitates the dispersion of a liquid by the formation of a spray. The production of a spray requires the fragmentation of liquid structures, such as liquid sheets or ligaments, into droplets, often by using kinetic energy to overcome the cost of creating additional surface area. A wide variety of spray nozzles exist, that make use of one or multiple liquid breakup mechanisms, which can be divided into three categories: liquid sheet breakup, jets and capillary waves. Spray nozzles are of great importance for many applications, where the spray nozzle is designed to have the right spray characteristics.
Dry lubricants or solid lubricants are materials that, despite being in the solid phase, are able to reduce friction between two surfaces sliding against each other without the need for a liquid oil medium.

Ultrasonic nozzles are a type of spray nozzle that use high frequency vibrations produced by piezoelectric transducers acting upon the nozzle tip that create capillary waves in a liquid film. Once the amplitude of the capillary waves reaches a critical height, they become too tall to support themselves and tiny droplets fall off the tip of each wave resulting in atomization.

Graphite oxide (GO), formerly called graphitic oxide or graphitic acid, is a compound of carbon, oxygen, and hydrogen in variable ratios, obtained by treating graphite with strong oxidizers and acids for resolving of extra metals. The maximally oxidized bulk product is a yellow solid with C:O ratio between 2.1 and 2.9, that retains the layer structure of graphite but with a much larger and irregular spacing.

Transparent conducting films (TCFs) are thin films of optically transparent and electrically conductive material. They are an important component in a number of electronic devices including liquid-crystal displays, OLEDs, touchscreens and photovoltaics. While indium tin oxide (ITO) is the most widely used, alternatives include wider-spectrum transparent conductive oxides (TCOs), conductive polymers, metal grids and random metallic networks, carbon nanotubes (CNT), graphene, nanowire meshes and ultra thin metal films.
Cladding is the bonding together of dissimilar metals. It is different from fusion welding or gluing as a method to fasten the metals together. Cladding is often achieved by extruding two metals through a die as well as pressing or rolling sheets together under high pressure.
Industrial porcelain enamel is the use of porcelain enamel for industrial, rather than artistic, applications. Porcelain enamel, a thin layer of ceramic or glass applied to a substrate of metal, is used to protect surfaces from chemical attack and physical damage, modify the structural characteristics of the substrate, and improve the appearance of the product.
A rapidly increasing list of graphene production techniques have been developed to enable graphene's use in commercial applications.

Boron nitride nanosheet is a two-dimensional crystalline form of the hexagonal boron nitride (h-BN), which has a thickness of one to few atomic layers. It is similar in geometry as well as physical and thermal properties to its all-carbon analog graphene, but has very different chemical and electronic properties – contrary to the black and highly conducting graphene, BN nanosheets are electrical insulators with a band gap of ~5.9 eV, and therefore appear white in color.
Graphene is the only form of carbon in which every atom is available for chemical reaction from two sides. Atoms at the edges of a graphene sheet have special chemical reactivity. Graphene has the highest ratio of edge atoms of any allotrope. Defects within a sheet increase its chemical reactivity. The onset temperature of reaction between the basal plane of single-layer graphene and oxygen gas is below 260 °C (530 K). Graphene combusts at 350 °C (620 K). Graphene is commonly modified with oxygen- and nitrogen-containing functional groups and analyzed by infrared spectroscopy and X-ray photoelectron spectroscopy. However, determination of structures of graphene with oxygen- and nitrogen- functional groups requires the structures to be well controlled.

Detonation spraying is one of the many forms of thermal spraying techniques that are used to apply a protective coating at supersonic velocities to a material in order to change its surface characteristics. This is primarily to improve the durability of a component. It was first invented in 1955 by H.B. Sargent, R.M. Poorman and H. Lamprey and is applied to a component using a specifically designed detonation gun (D-gun). The component being sprayed must be prepared correctly by removing all surface oils, greases, debris and roughing up the surface in order to achieve a strongly bonded detonation spray coating. This process involves the highest velocities and temperatures (≈4000 °C) of coating materials compared to all other forms of thermal spraying techniques. Which means detonation spraying is able to apply low porous and low oxygen content protective coatings that protect against corrosion, abrasion and adhesion under low load.
Cold spray additive manufacturing (CSAM) is a particular application of cold spraying, able to fabricate freestanding parts or to build features on existing components. During the process, fine powder particles are accelerated in a high-velocity compressed gas stream, and upon the impact on a substrate or backing plate, deform and bond together creating a layer. Moving the nozzle over a substrate repeatedly, a deposit is building up layer-by-layer, to form a part or component. If an industrial robot or computer controlled manipulator controls the spray gun movements, complex shapes can be created. To achieve 3D shape, there are two different approaches. First to fix the substrate and move the cold spray gun/nozzle using a robotic arm, the second one is to move the substrate with a robotic arm, and keep the spray-gun nozzle fixed. There is also a possibility to combine these two approaches either using two robotic arms or other manipulators. The process always requires a substrate and uses only powder as raw material.
References
- 1 2 3 Galatzer-Levy, Jeanne. "Supersonic spray delivers high-quality graphene layer". uic.edu/uic/. The Board of Trustees of the University of Illinois. Retrieved 30 October 2014.
- 1 2 de La Fuente, Jesus. "Graphene Applications". Graphenea. Retrieved 9 November 2014.
- 1 2 3 4 Kim, Do-Yeon (2014). "Self-Healing Reduced Graphene Oxide Films by Supersonic Kinetic Spraying". Advanced Functional Materials. John Wiley & Sons, Inc. 24 (31): 4986–4995. doi:10.1002/adfm.201400732.
- 1 2 3 Solon, Olivia. "Supersonic spray gun produces 'flawless' layer of graphene". Wired. Condé Nast UK. Retrieved 29 October 2014.
- 1 2 Anthony, Sebastian. "Researchers create high-quality graphene with shockingly simple supersonic spray system". extremetech.com. Ziff Davis . Retrieved 29 October 2014.
- 1 2 3 Hayes, Caroline (2019-06-21). "Graphene: what is it good for?". eandt.theiet.org. Retrieved 2020-02-13.
- ↑ "Graphene coating". coating.ca.
- 1 2 "Graphene Coatings: Exciting Properties and Wide-Ranging Potential". American Coatings Association. Retrieved 2020-02-13.
- ↑ M., Topsakal; H., Şahin; C., Ciraci (23 April 2012). "Graphene coatings: An efficient protection from oxidation". Physical Review. 85 (15): 155445. arXiv: 1203.2580 . Bibcode:2012PhRvB..85o5445T. doi:10.1103/PhysRevB.85.155445.