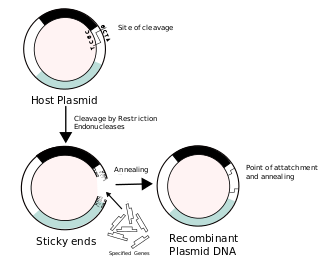
Recombinant DNA (rDNA) molecules are DNA molecules formed by laboratory methods of genetic recombination that bring together genetic material from multiple sources, creating sequences that would not otherwise be found in the genome.
Pharming, a portmanteau of "farming" and "pharmaceutical", refers to the use of genetic engineering to insert genes that code for useful pharmaceuticals into host animals or plants that would otherwise not express those genes, thus creating a genetically modified organism (GMO). Pharming is also known as molecular farming, molecular pharming or biopharming.

Biocon Limited is an Indian biopharmaceutical company based in Bangalore. It was founded by Kiran Mazumdar-Shaw in 1978. The company manufactures generic active pharmaceutical ingredients (APIs) that are sold in approximately 120 countries, including the United States and Europe. It also manufactures novel biologics as well as biosimilar insulins and antibodies, which are sold in India as branded formulations. Biocon's biosimilar products are also sold in both bulk and formulation forms in several emerging markets.
Baxter International Inc. is an American multinational healthcare company with headquarters in Deerfield, Illinois.
A biopharmaceutical, also known as a biological medical product, or biologic, is any pharmaceutical drug product manufactured in, extracted from, or semisynthesized from biological sources. Different from totally synthesized pharmaceuticals, they include vaccines, whole blood, blood components, allergenics, somatic cells, gene therapies, tissues, recombinant therapeutic protein, and living medicines used in cell therapy. Biologics can be composed of sugars, proteins, nucleic acids, or complex combinations of these substances, or may be living cells or tissues. They are isolated from living sources—human, animal, plant, fungal, or microbial. They can be used in both human and animal medicine.
The pharmaceutical industry in India was valued at an estimated US$42 billion in 2021 and is estimated to reach $130 billion by 2030. India is the world's largest provider of generic medicines by volume, with a 20% share of total global pharmaceutical exports. It is also the largest vaccine supplier in the world by volume, accounting for more than 60% of all vaccines manufactured in the world. Indian pharmaceutical products are exported to various regulated markets including the US, UK, European Union and Canada.
The Center of Molecular Immunology or CIM, is a cancer research institution located on the west side of Havana, Cuba. 23.0755°N 82.4708°W
Agenus Inc. is a Lexington, Massachusetts-based biotechnology company focused on immunotherapy including immuno-oncology, a field that uses the immune system to control or cure cancer. The company is developing checkpoint modulators (CPMs), patient-specific anti-cancer vaccines, and adjuvants desugned for use with various vaccines. CPM development is a particularly fast-moving field, since early products have produced unprecedented clinical benefits for patients.

Factor VIII is a medication used to treat and prevent bleeding in people with hemophilia A and other causes of low factor VIII. Certain preparations may also be used in those with von Willebrand's disease. It is given by slow injection into a vein.

Sartorius AG is an international pharmaceutical and laboratory equipment supplier, covering the segments of Bioprocess Solutions and Lab Products & Services. In September 2021, Sartorius has been admitted to the DAX, Germany's largest stock market index. As a leading partner to the biopharmaceutical research and industry, Sartorius supports its customers in the development and production of biotech drugs and vaccines - from the initial idea in the laboratory to commercial production. Sartorius conducts its operating business in the two divisions Bioprocess Solutions and Lab Products&Services. The divisions bundle their respective businesses according to the same application areas and customer groups. The divisions share some of the infrastructure and central services.
A subunit vaccine is a vaccine that contains purified parts of the pathogen that are antigenic, or necessary to elicit a protective immune response. Subunit vaccine can be made from dissembled viral particles in cell culture or recombinant DNA expression, in which case it is a recombinant subunit vaccine.
MorphoSys AG is a biopharmaceutical company founded in 1992. The company is headquartered near Munich, Germany and has a wholly owned subsidiary, MorphoSys US Inc., in Boston MA in the US. The company has various antibody, protein and peptide technologies that it uses to discover and develop both proprietary and partnered drug candidates. The company has more than 100 drugs in its wider pipeline that are being investigated for a variety of diseases. While many of these are being developed in partnership with pharma and biotech companies, MorphoSys also has a proprietary pipeline with a focus on cancer and autoimmune diseases.
Passive immunization is a medical strategy long employed to provide temporary protection against pathogens. Early implementations involved recovering ostensibly cell-free plasma from the blood of human survivors or from non-human animals deliberately exposed to a specific pathogen or toxin. These approaches resulted in crude purifications of plasma-soluble proteins including antibodies.
Eurogentec is an international biotechnology supplier, based in Belgium, that specializes in genomics and proteomics kits and reagents as well as cGMP biologics. The company was founded in 1985 as a spin-off from the University of Liège. Eurogentec's contract manufacturing organization facilities are licensed by the Belgian Ministry of Health to produce clinical trial and commercial biopharmaceutical material and also licensed by the US FDA to manufacture a commercial recombinant protein product for the US market. Eurogentec operates two manufacturing facilities in Belgium that provide custom biologics and oligonucleotide-based components for diagnostic and therapeutic/prophylactic applications.
SAV001-H is the first candidate preventive HIV vaccine using a killed or "dead" version of the HIV-1 virus.

Sutro Biopharma, Inc. is a public biotechnology company headquartered in South San Francisco, California focused on clinical-stage drug discovery, development and manufacturing. Using a proprietary cell-free protein synthesis platform, Sutro is working on oncology therapeutics using protein engineering and rational design. Founded in 2003 under the name Fundamental Applied Biology, the company name changed to Sutro Biopharma in 2009. The current CEO, William Newell, joined Sutro in January 2009.

Samsung Biologics is a South Korean biotechnology company headquartered in Songdo, Incheon, South Korea. The biotech division of Samsung Group, it provides contract development and manufacturing (CDMO) services to the biopharmaceutical industry.
Host cell proteins (HCPs) are process-related protein impurities that are produced by the host organism during biotherapeutic manufacturing and production. During the purification process, a majority of produced HCPs are removed from the final product. However, residual HCPs still remain in the final distributed pharmaceutical drug. Examples of HCPs that may remain in the desired pharmaceutical product include: monoclonal antibodies (mAbs), antibody-drug-conjugates (ADCs), therapeutic proteins, vaccines, and other protein-based biopharmaceuticals.

ZF2001, trade-named Zifivax or ZF-UZ-VAC-2001, is an adjuvanted protein subunit COVID-19 vaccine developed by Anhui Zhifei Longcom in collaboration with the Institute of Microbiology at the Chinese Academy of Sciences. The vaccine candidate is in Phase III trials with 29,000 participants in China, Ecuador, Malaysia, Pakistan, and Uzbekistan.

SCB-2019 is a protein subunit COVID-19 vaccine developed by Clover Biopharmaceuticals using an adjuvant from Dynavax technologies. Positive results of Phase I trials for the vaccine were published in The Lancet and the vaccine completed enrollment of 29,000 participants in Phase II/III trials in July 2021. In September 2021, SCB-2019 announced Phase III results showing 67% efficacy against all cases of COVID-19 and 79% efficacy against all cases of the Delta variant. Additionally, the vaccine was 84% effective against moderate cases and 100% effective against hospitalization.






