Atrial natriuretic peptide (ANP) or atrial natriuretic factor (ANF) is a natriuretic peptide hormone secreted from the cardiac atria that in humans is encoded by the NPPA gene. Natriuretic peptides are a family of hormone/paracrine factors that are structurally related. The main function of ANP is causing a reduction in expanded extracellular fluid (ECF) volume by increasing renal sodium excretion. ANP is synthesized and secreted by cardiac muscle cells in the walls of the atria in the heart. These cells contain volume receptors which respond to increased stretching of the atrial wall due to increased atrial blood volume.

Guanylate cyclase is a lyase enzyme that converts guanosine triphosphate (GTP) to cyclic guanosine monophosphate (cGMP) and pyrophosphate:

Vasoactive intestinal peptide, also known as vasoactive intestinal polypeptide or VIP, is a peptide hormone that is vasoactive in the intestine. VIP is a peptide of 28 amino acid residues that belongs to a glucagon/secretin superfamily, the ligand of class II G protein–coupled receptors. VIP is produced in many tissues of vertebrates including the gut, pancreas, cortex, and suprachiasmatic nuclei of the hypothalamus in the brain. VIP stimulates contractility in the heart, causes vasodilation, increases glycogenolysis, lowers arterial blood pressure and relaxes the smooth muscle of trachea, stomach and gallbladder. In humans, the vasoactive intestinal peptide is encoded by the VIP gene.

Guanylate cyclase 2C, also known as guanylyl cyclase C (GC-C), intestinal guanylate cyclase, guanylate cyclase-C receptor, or the heat-stable enterotoxin receptor (hSTAR) is an enzyme that in humans is encoded by the GUCY2C gene.

Glucagon-like peptide-1 (GLP-1) is a 30- or 31-amino-acid-long peptide hormone deriving from the tissue-specific posttranslational processing of the proglucagon peptide. It is produced and secreted by intestinal enteroendocrine L-cells and certain neurons within the nucleus of the solitary tract in the brainstem upon food consumption. The initial product GLP-1 (1–37) is susceptible to amidation and proteolytic cleavage, which gives rise to the two truncated and equipotent biologically active forms, GLP-1 (7–36) amide and GLP-1 (7–37). Active GLP-1 protein secondary structure includes two α-helices from amino acid position 13–20 and 24–35 separated by a linker region.

Peptide YY (PYY), also known as peptide tyrosine tyrosine, is a peptide that in humans is encoded by the PYY gene. Peptide YY is a short peptide released from cells in the ileum and colon in response to feeding. In the blood, gut, and other elements of periphery, PYY acts to reduce appetite; similarly, when injected directly into the central nervous system, PYY is also anorexigenic, i.e., it reduces appetite.
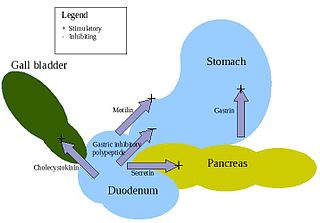
Enteroendocrine cells are specialized cells of the gastrointestinal tract and pancreas with endocrine function. They produce gastrointestinal hormones or peptides in response to various stimuli and release them into the bloodstream for systemic effect, diffuse them as local messengers, or transmit them to the enteric nervous system to activate nervous responses. Enteroendocrine cells of the intestine are the most numerous endocrine cells of the body. They constitute an enteric endocrine system as a subset of the endocrine system just as the enteric nervous system is a subset of the nervous system. In a sense they are known to act as chemoreceptors, initiating digestive actions and detecting harmful substances and initiating protective responses. Enteroendocrine cells are located in the stomach, in the intestine and in the pancreas. Microbiota play key roles in the intestinal immune and metabolic responses in these enteroendocrine cells via their fermentation product, acetate.

Pituitary adenylate cyclase-activating polypeptide also known as PACAP is a protein that in humans is encoded by the ADCYAP1 gene. pituitary adenylate cyclase-activating polypeptide is similar to vasoactive intestinal peptide. One of its effects is to stimulate enterochromaffin-like cells. It binds to vasoactive intestinal peptide receptor and to the pituitary adenylate cyclase-activating polypeptide receptor.

The gastrin-releasing peptide receptor (GRPR), now properly known as BB2 is a G protein-coupled receptor whose endogenous ligand is gastrin releasing peptide. In humans it is highly expressed in the pancreas and is also expressed in the stomach, adrenal cortex and brain.

Pituitary adenylate cyclase-activating polypeptide type I receptor also known as PAC1, is a protein that in humans is encoded by the ADCYAP1R1 gene. This receptor binds pituitary adenylate cyclase activating peptide.

Free fatty acid receptor 2 (FFAR2), also known as G-protein coupled receptor 43 (GPR43), is a rhodopsin-like G-protein coupled receptor (GPCR) encoded by the FFAR2 gene. In humans, the FFAR2 gene is located on the long arm of chromosome 19 at position 13.12 (19q13.12).

Neuropeptide Y receptor type 2 (Y2R) is a member of the neuropeptide Y receptor family of G-protein coupled receptors, that in humans is encoded by the NPY2R gene.

Vasoactive intestinal peptide receptor 2 also known as VPAC2, is a G-protein coupled receptor that in humans is encoded by the VIPR2 gene.
Vasoactive intestinal polypeptide receptor 1 also known as VPAC1, is a protein, that in humans is encoded by the VIPR1 gene. VPAC1 is expressed in the brain (cerebral cortex, hippocampus, amygdala), lung, prostate, peripheral blood leukocytes, liver, small intestine, heart, spleen, placenta, kidney, thymus and testis.

Leucine-rich repeat-containing G-protein coupled receptor 5 (LGR5) also known as G-protein coupled receptor 49 (GPR49) or G-protein coupled receptor 67 (GPR67) is a protein that in humans is encoded by the LGR5 gene. It is a member of GPCR class A receptor proteins. R-spondin proteins are the biological ligands of LGR5. LGR5 is expressed across a diverse range of tissue such as in the muscle, placenta, spinal cord and brain and particularly as a biomarker of adult stem cells in certain tissues.

Natriuretic peptide receptor C/guanylate cyclase C , also known as NPR3, is an atrial natriuretic peptide receptor. In humans it is encoded by the NPR3 gene.

Guanylyl cyclase-activating protein 2 is an enzyme that in humans is encoded by the GUCA1B gene. Alternative names:

Uroguanylin is a 16 amino acid peptide that is secreted by enterochromaffin cells in the duodenum and proximal small intestine. Guanylin acts as an agonist of the guanylyl cyclase receptor guanylate cyclase 2C (GC-C), and regulates electrolyte and water transport in intestinal and renal epithelia. By agonizing this guanylyl cyclase receptor, uroguanylin and guanylin cause intestinal secretion of chloride and bicarbonate to dramatically increase; this process is helped by the second messenger cGMP. Its sequence is H-Asn-Asp-Asp-Cys(1)-Glu-Leu-Cys(2)-Val-Asn-Val-Ala-Cys(1)-Thr-Gly-Cys(2)-Leu-OH.
hPG80 refers to the extracellular and oncogenic version of progastrin. This name first appeared in a scientific publication in January 2020. Until that date, scientific publications only mention 'progastrin', without necessarily explicitly specifying whether it is intracellular or extracellular in the tumor pathological setting.
Scott A. Waldman is an MD and biomedical scientist at Sidney Kimmel Medical College of Thomas Jefferson University, where he is the Samuel M.V. Hamilton Professor of Medicine, and also tenured professor and chair of the Department of Pharmacology & Experimental Therapeutics. He is author of a pharmacology textbook, and former chief editor of Clinical Pharmacology & Therapeutics. He is known for his work in atrial natriuretic factor intracellular signaling through guanylate cyclase (GC), and the relation of Guanylyl cyclase C (GC-C) to the pathogenesis of colorectal cancer. Also for his hypotheses concerning the roles of intestinal paracrine hormones in satiety, obesity and cancer risk. Waldman also holds a concurrent position as adjunct professor at the University of Delaware, School of Health Sciences.

















