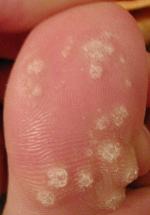
Warts are non-cancerous viral growths usually occurring on the hands and feet but can also affect other locations, such as the genitals or face. One or many warts may appear. They are distinguished from cancerous tumors as they are caused by a viral infection, such as a human papillomavirus, rather than a cancerous growth.

The Papanicolaou test is a method of cervical screening used to detect potentially precancerous and cancerous processes in the cervix or, more rarely, anus. Abnormal findings are often followed up by more sensitive diagnostic procedures and, if warranted, interventions that aim to prevent progression to cervical cancer. The test was independently invented in the 1920s by the Greek physician Georgios Papanikolaou and named after him. A simplified version of the test was introduced by the Canadian obstetrician Anna Marion Hilliard in 1957.

Cervical cancer is a cancer arising from the cervix. It is due to the abnormal growth of cells that have the ability to invade or spread to other parts of the body. Early on, typically no symptoms are seen. Later symptoms may include abnormal vaginal bleeding, pelvic pain or pain during sexual intercourse. While bleeding after sex may not be serious, it may also indicate the presence of cervical cancer.
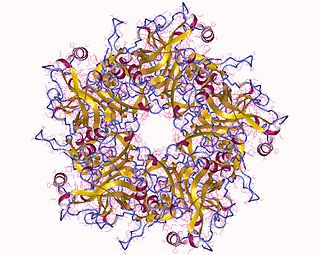
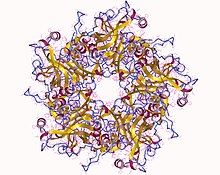
Human papillomavirus infection is caused by a DNA virus from the Papillomaviridae family. Many HPV infections cause no symptoms and 90% resolve spontaneously within two years. In some cases, an HPV infection persists and results in either warts or precancerous lesions. These lesions, depending on the site affected, increase the risk of cancer of the cervix, vulva, vagina, penis, anus, mouth, tonsils, or throat. Nearly all cervical cancer is due to HPV, and two strains – HPV16 and HPV18 – account for 70% of all cases. HPV16 is responsible for almost 90% of HPV-positive oropharyngeal cancers. Between 60% and 90% of the other cancers listed above are also linked to HPV. HPV6 and HPV11 are common causes of genital warts and laryngeal papillomatosis.

Anal cancer is a cancer which arises from the anus, the distal opening of the gastrointestinal tract. Symptoms may include bleeding from the anus or a lump near the anus. Other symptoms may include pain, itchiness, or discharge from the anus. A change in bowel movements may also occur.

A Langerhans cell (LC) is a tissue-resident macrophage of the skin once thought to be a resident dendritic cell. These cells contain organelles called Birbeck granules. They are present in all layers of the epidermis and are most prominent in the stratum spinosum. They also occur in the papillary dermis, particularly around blood vessels, as well as in the mucosa of the mouth, foreskin, and vaginal epithelium. They can be found in other tissues, such as lymph nodes, particularly in association with the condition Langerhans cell histiocytosis (LCH).

A papilloma is a benign epithelial tumor growing exophytically in nipple-like and often finger-like fronds. In this context, papilla refers to the projection created by the tumor, not a tumor on an already existing papilla.

Cervical intraepithelial neoplasia (CIN), also known as cervical dysplasia, is the abnormal growth of cells on the surface of the cervix that could potentially lead to cervical cancer. More specifically, CIN refers to the potentially precancerous transformation of cells of the cervix.

Human papillomavirus (HPV) vaccines are vaccines that prevent infection by certain types of human papillomavirus (HPV). Available HPV vaccines protect against either two, four, or nine types of HPV. All HPV vaccines protect against at least HPV types 16 and 18, which cause the greatest risk of cervical cancer. It is estimated that HPV vaccines may prevent 70% of cervical cancer, 80% of anal cancer, 60% of vaginal cancer, 40% of vulvar cancer, and show more than 90% efficacy in preventing HPV-positive oropharyngeal cancers. They additionally prevent some genital warts, with the quadrivalent and nonavalent vaccines that protect against HPV types HPV-6 and HPV-11 providing greater protection.

Gardasil is an HPV vaccine for use in the prevention of certain strains of human papillomavirus (HPV). It was developed by Merck & Co. High-risk human papilloma virus (hr-HPV) genital infection is the most common sexually transmitted infection among women. The HPV strains that Gardasil protects against are sexually transmitted, specifically HPV types 6, 11, 16 and 18. HPV types 16 and 18 cause an estimated 70% of cervical cancers, and are responsible for most HPV-induced anal, vulvar, vaginal, and penile cancer cases. HPV types 6 and 11 cause an estimated 90% of genital warts cases. HPV type 16 is responsible for almost 90% of HPV-positive oropharyngeal cancers, and the prevalence is higher in males than females. Though Gardasil does not treat existing infection, vaccination is still recommended for HPV-positive individuals, as it may protect against one or more different strains of the disease.
Cervarix is a vaccine against certain types of cancer-causing human papillomavirus (HPV).
Margaret Anne Stanley, OBE FMedSc, is a British virologist and epithelial biologist. She attended the Universities of London, Bristol, and Adelaide. As of 2018, she is an Emeritus Professor of Epithelial Biology in the Department of Pathology at the University of Cambridge and a Fellow of the Academy of Medical Sciences. She is also an Honorary Fellow of the UK Royal College of Obstetricians and Gynaecologists and an honorary fellow of Christ's College, Cambridge. Stanley is a research scientist in virology focusing on the human papillomavirus (HPV). Her research work has led to new scientific findings on HPV. Additionally, she uses her expertise on HPV to serve on multiple advisory committees and journal editorial boards.

Oropharyngeal cancer, also known as oropharyngeal squamous cell carcinoma and tonsil cancer, is a disease in which abnormal cells with the potential to both grow locally and spread to other parts of the body are found in the oral cavity, in the tissue of the part of the throat (oropharynx) that includes the base of the tongue, the tonsils, the soft palate, and the walls of the pharynx.

Cervical cancer screening is a medical screening test designed to identify risk of cervical cancer. Cervical screening may involve looking for viral DNA, and/or to identify abnormal, potentially precancerous cells within the cervix as well as cells that have progressed to early stages of cervical cancer. One goal of cervical screening is to allow for intervention and treatment so abnormal lesions can be removed prior to progression to cancer. An additional goal is to decrease mortality from cervical cancer by identifying cancerous lesions in their early stages and providing treatment prior to progression to more invasive disease.
Diane Medved Harper is a United States professor in the Department of Family Medicine at the University of Michigan. Her area of expertise is human papillomavirus (HPV) and the diseases associated with it, as well as colposcopy, and she was one of the investigators in the clinical trials of Gardasil and Cervarix, vaccines against HPV.
Human papillomavirus (HPV)-associated oropharyngeal cancer awareness and prevention is a vital concept from a public and community health perspective.

Gynecologic cancer disparities in the United States refer to differences in incidence, prevalence, and mortality from gynecologic cancers between population groups. The five main types of gynecologic cancer include cervical cancer, ovarian cancer, endometrial cancer, vaginal cancer, and vulvar cancer. For patients with these and other gynecologic malignancies within the United States, disparities across the care continuum by socioeconomic status and racial/ethnic background have been previously identified and studied. The causes behind these disparities are multifaceted and a complex interplay of systemic differences in health as well as individual patient factors such as cultural, educational, and economic barriers.
Karen Canfell is an Australian epidemiologist and cancer researcher.

Human Papillomavirus in Ghana; each year about 3,000 Ghanaian women are diagnosed cervical cancer caused by Human Papillomavirus, HPV. It is estimated that 2,000 women die out of the 3000 annually.
The Oral Cancer Foundation, sometimes abbreviated to OCF, is an American, IRS-registered, 501(c)(3) non-profit organization, which focuses on oral and oropharyngeal cancer related issues and public awareness of the disease.