Related Research Articles

An HIV vaccine may have the purpose of protecting individuals who do not have HIV from being infected with the virus, or treating an HIV-infected person. There are two approaches to an HIV vaccine: an active vaccination approach in which a vaccine aims to induce an immune response against HIV; and a passive vaccination approach in which preformed antibodies against HIV are administered.

Microbicides for sexually transmitted diseases are pharmacologic agents and chemical substances that are capable of killing or destroying certain microorganisms that commonly cause human infection.

The HIV Prevention Trials Network (HPTN) is a worldwide collaborative clinical trials network that brings together investigators, ethicists, community and other partners to develop and test the safety and efficacy of interventions designed to prevent the acquisition and transmission of HIV. HPTN studies evaluate new HIV prevention interventions and strategies in populations and geographical regions that bear a disproportionate burden of infection. The HPTN is committed to the highest ethical standards for its clinical trials and recognizes the importance of community engagement in all phases of the research process.

The HIV Vaccine Trials Network (HVTN) is a non-profit organization which connects physicians and scientists with activists and community educators for the purpose of conducting clinical trials seeking a safe and effective HIV vaccine. Collaboratively, researchers and laypeople review potential vaccines for safety, immune response, and efficacy. The HVTN is a network for testing vaccines, and while its members may also work in vaccine development for other entities, the mission of the HVTN does not include vaccine design.
AIDSVAX is an experimental HIV vaccine that was developed originally at Genentech in San Francisco, California, and later tested by the VaxGen company, a Genentech offshoot. The development and trials of the vaccine received significant coverage in the international media, but American trials proved inconclusive. The vaccine was then tested on a group of at-risk individuals in Thailand.
Lawrence Corey is professor of Medicine and Laboratory Medicine at the University of Washington, a member of the Vaccine and Infectious Disease Division and past president and director of Fred Hutchinson Cancer Research Center in Seattle, Washington. He also serves as the founding director and co-principal investigator of the HIV Vaccine Trials Network. The American physician-scientist is an internationally recognized expert in virology, immunology and vaccine development. His discoveries over the past 30 years are cited as having deepened the understanding of both the pathogenesis and treatment of diseases caused by viruses, especially human immunodeficiency virus (HIV) and herpes viruses. Corey has pioneered some of the most significant advances in the creation of safe and effective antivirals for herpes viruses and HIV, the testing of experimental vaccines for HIV and genital herpes, and the designing of new methods for diagnosing and monitoring therapies for viral infections.
GeoVax is a biotechnology company established primarily to develop an effective and safe vaccine against HIV-1. Ultimately the company is to create vaccines for many serious human diseases for which none currently exist. GeoVax is currently conducting multiple site Phase 2 Human clinical trials for HIV/AIDS preventive vaccine products following successful completion of multiple Phase 1 human clinical trials.
PRO 140 (leronlimab) is a humanized monoclonal antibody targeted against the CCR5 receptor found on T lymphocytes of the human immune system. It is being investigated as a potential therapy in the treatment of HIV infection. The United States Food and Drug Administration has designated PRO 140 for fast-track approval. In February 2008, the drug entered Phase 2 clinical trials and a phase 3 trial was begun in 2015. In February 2018, Cytodyn Inc reported that the primary endpoint had been achieved in the PRO 140 pivotal combination therapy trial in HIV infection. Pro 140 is one of several antiviral drugs being tested for coronavirus disease 2019.
The United States Military HIV Research Program was initiated by the United States Congress in 1986, in reaction to the threat of lost effectiveness of U.S./Allied troops due to HIV infection. The mission of MHRP is to develop an HIV-1 vaccine, provide prevention, care, and treatment, and conduct meaningful HIV/AIDS research for the global community through the President's Emergency Plan for AIDS Relief (PEPFAR). It is centered at the Walter Reed Army Institute of Research (WRAIR), and has established five international research sites in Africa and Asia. MHRP also partners with the Armed Forces Research Institute of Medical Sciences (AFRIMS) in Thailand. MHRP works closely with The Henry M. Jackson Foundation for the Advancement of Military Medicine (HJF), most notably in the development of the RV144 HIV vaccine in Thailand. MHRP is the largest research program supported by the HJF.
The STEP Study and Phambili Trial were clinical trials which both tested the efficacy of a recombinant adenovirus 5 vector HIV vaccine. STEP was the second HIV vaccine efficacy trial ever conducted. Vaccination in both trials stopped before the trials were scheduled to finish, when the data safety monitoring board reviewed data from the STEP study and found that the vaccine was not preventing HIV infection.
HVTN 505 is a clinical trial testing an HIV vaccine regimen on research participants. The trial is conducted by the HIV Vaccine Trials Network and sponsored by the National Institute of Allergy and Infectious Diseases. Vaccinations were stopped in April 2013 due to initial results showing that the vaccine was ineffective in preventing HIV infections and lowering viral load among those participants who had become infected with HIV. All study participants will continue to be monitored for safety and any long-term effects.
RTS,S/AS01 is a recombinant protein-based malaria vaccine.

The Office of HIV/AIDS Network Coordination, known as HANC, works with the National Institutes of Health HIV/AIDS clinical trials networks with the intent of creating a more integrated, collaborative and flexible research structure. The networks are an affiliated group of national and international medical research institutions and investigators that conduct clinical HIV/AIDS research to develop safe and effective drugs, prevention strategies, and vaccines.

HIV/AIDS research includes all medical research that attempts to prevent, treat, or cure HIV/AIDS, as well as fundamental research about the nature of HIV as an infectious agent and AIDS as the disease caused by HIV.
Novavax, Inc. is a clinical-stage vaccine company headquartered in Gaithersburg, Maryland with additional facilities in Rockville, Maryland and Uppsala, Sweden. Novavax received grants from Bill Gates in an effort to help with global health crisis. It has an ongoing Phase 3 clinical trial in older adults for its candidate vaccine, NanoFlu. The company positions NanoFlu for the urgent unmet medical need for a more effective vaccine against influenza, particularly in the older adult population which often experiences serious and sometimes life-threatening complications. In Jan 2020, it was granted Fast Track Designation by the U.S. Food and Drug Administration (FDA) for NanoFlu.
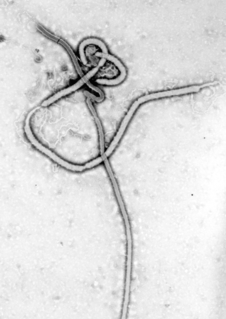
Recombinant vesicular stomatitis virus–Zaire Ebola virus (rVSV-ZEBOV), sold under the brand name Ervebo is a vaccine that prevents Ebola. When used in ring vaccination, rVSV-EBOV has shown a high level of protection. Around half the people given the vaccine have mild to moderate adverse effects that include headache, fatigue, and muscle pain.

Ebola vaccines are a number of vaccines to prevent Ebola that are either approved or in development. The first vaccine to be approved in the United States was rVSV-ZEBOV in December of 2019. It had been used extensively in 2018-19 under a compassionate use protocol. Several promising vaccine candidates have been shown to protect nonhuman primates against lethal infection.

Joshua W. Robbins is an American HIV/AIDS activist, blogger, talent agent, writer, and social media marketer. His work has been featured on The Advocate, Human Rights Campaign, Healthline, POZ and a myriad of additional publications. Robbins was named to the POZ 100 List in 2013.
M. Juliana “Julie” McElrath is a senior vice president and director of the vaccine and infectious disease division at Fred Hutchinson Cancer Research Center and principal investigator of the HIV Vaccine Trials Network Laboratory Center in Seattle, Washington. She also is a professor at the University of Washington.
A Zika virus vaccine is designed to prevent the symptoms and complications of Zika virus infection in humans. As Zika virus infection of pregnant women may result in congenital defects in the newborn, the vaccine will attempt to protect against congenital Zika syndrome during the current or any future outbreak. As of April 2019, no vaccines have been approved for clinical use, however a number of vaccines are currently in clinical trials. The goal of a Zika virus vaccine is to elicit protective antibodies against the Zika virus to prevent infection and severe disease. The challenges in developing a safe and effective vaccine include limiting side effects such as Guillain-Barré syndrome, a potential consequence of Zika virus infection. Additionally, as dengue virus is closely related to Zika virus, the vaccine needs to minimize the possibility of antibody-dependent enhancement of dengue virus infection.
References
- ↑ Doughton, Sandi (3 February 2020). "'Incredibly disappointing': Fred Hutch, feds end HIV vaccine trial after data shows no benefit". The Seattle Times .
- ↑ "Experimental HIV vaccine regimen ineffective in preventing HIV". National Institutes of Health (NIH). 3 February 2020.
- ↑ Russell, Sabin (4 February 2020). "Promising HIV vaccine fails to show efficacy, trial halted". Fred Hutch.
- ↑ Fitzsimons, Tim (1 December 2019). "HIV vaccine in 2021? Leading experts 'optimistic' about ongoing trials". NBC News.
- ↑ Lee, Bruce Y. (3 December 2019). "An HIV Vaccine By 2021? Here Is What Needs To Happen". Forbes.
- ↑ "New HIV vaccine trial to start in South Africa". www.cbsnews.com. 28 November 2016.
- ↑ Galeon, Dom (29 November 2016). "An End to AIDS: New Vaccine Could Be The "Final Nail in the Coffin" for HIV". Futurism.
- ↑ "HIV vaccine trial begins in South Africa". BBC News. 30 November 2016.
- ↑ Cairns, Gus (26 July 2017). "Another HIV vaccine efficacy trial will start this year". Aidsmap .