
The human immunodeficiency viruses (HIV) are two species of Lentivirus that infect humans. Over time, they cause acquired immunodeficiency syndrome (AIDS), a condition in which progressive failure of the immune system allows life-threatening opportunistic infections and cancers to thrive. Without treatment, average survival time after infection with HIV is estimated to be 9 to 11 years, depending on the HIV subtype. In most cases, HIV is a sexually transmitted infection and occurs by contact with or transfer of blood, pre-ejaculate, semen, and vaginal fluids. Research has shown that HIV is untransmittable through condomless sexual intercourse if the HIV-positive partner has a consistently undetectable viral load. Non-sexual transmission can occur from an infected mother to her infant during pregnancy, during childbirth by exposure to her blood or vaginal fluid, and through breast milk. Within these bodily fluids, HIV is present as both free virus particles and virus within infected immune cells.

Adenoviruses are medium-sized, nonenveloped viruses with an icosahedral nucleocapsid containing a double stranded DNA genome. Their name derives from their initial isolation from human adenoids in 1953.

Feline immunodeficiency virus (FIV) is a Lentivirus that affects cats worldwide, with 2.5% to 4.4% of felines being infected. FIV differs taxonomically from two other feline retroviruses, feline leukemia virus (FeLV) and feline foamy virus (FFV), and is more closely related to human immunodeficiency virus (HIV). Within FIV, five subtypes have been identified based on nucleotide sequence differences coding for the viral envelope (env) or polymerase (pol). FIV is the only non-primate lentivirus to cause an AIDS-like syndrome, but FIV is not typically fatal for cats, as they can live relatively healthily as carriers and transmitters of the disease for many years. A vaccine is available, although its efficacy remains uncertain. Cats will test positive for FIV antibodies after vaccination.
The genome and proteins of HIV have been the subject of extensive research since the discovery of the virus in 1983. "In the search for the causative agent, it was initially believed that the virus was a form of the Human T-cell leukemia virus (HTLV), which was known at the time to affect the human immune system and cause certain leukemias. However, researchers at the Pasteur Institute in Paris isolated a previously unknown and genetically distinct retrovirus in patients with AIDS which was later named HIV." Each virion comprises a viral envelope and associated matrix enclosing a capsid, which itself encloses two copies of the single-stranded RNA genome and several enzymes. The discovery of the virus itself occurred two years following the report of the first major cases of AIDS-associated illnesses.

Envelope glycoprotein GP120 is a glycoprotein exposed on the surface of the HIV envelope. It was discovered by Professors Tun-Hou Lee and Myron "Max" Essex of the Harvard School of Public Health in 1988. The 120 in its name comes from its molecular weight of 120 kDa. Gp120 is essential for virus entry into cells as it plays a vital role in attachment to specific cell surface receptors. These receptors are DC-SIGN, Heparan Sulfate Proteoglycan and a specific interaction with the CD4 receptor, particularly on helper T-cells. Binding to CD4 induces the start of a cascade of conformational changes in gp120 and gp41 that lead to the fusion of the viral membrane with the host cell membrane. Binding to CD4 is mainly electrostatic although there are van der Waals interactions and hydrogen bonds.
HIV superinfection is a condition in which a person with an established human immunodeficiency virus infection acquires a second strain of HIV, often of a different subtype. These can form a recombinant strain that co-exists with the strain from the initial infection, as well from reinfection with a new virus strain, and may cause more rapid disease progression or carry multiple resistances to certain HIV medications.
AIDSVAX is an experimental HIV vaccine that was developed originally at Genentech in San Francisco, California, and later tested by the VaxGen company, a Genentech offshoot. The development and trials of the vaccine received significant coverage in the international media, but American trials proved inconclusive. The vaccine was then tested on a group of at-risk individuals in Thailand.

Antibody-dependent enhancement (ADE), sometimes less precisely called immune enhancement or disease enhancement, is a phenomenon in which binding of a virus to suboptimal antibodies enhances its entry into host cells, followed by its replication. Antiviral antibodies promote viral infection of target immune cells by exploiting the phagocytic FcγR or complement pathway. After interaction with the virus the antibody binds Fc receptors (FcR) expressed on certain immune cells or some of the complement proteins. FcγR binds antibody via its fragment crystallizable region (Fc). Usually the process of phagocytosis is accompanied by the virus degradation, however, if the virus is not neutralized, antibody binding might result in a virus escape and therefore, enhanced infection. Thus, phagocytosis can cause viral replication, with the subsequent death of immune cells. The virus “deceives” the process of phagocytosis of immune cells and uses the host's antibodies as a Trojan horse. ADE may occur due to the non-neutralizing characteristic of the antibody, which bind viral epitopes other than those involved in a host cell attachment and entry. ADE may also happen due to the presence of sub-neutralizing concentrations of antibodies. In addition ADE can be induced when the strength of antibody-antigen interaction is below the certain threshold. This phenomenon might lead to both increased virus infectivity and virulence. The viruses that can cause ADE frequently share some common features such as antigenic diversity, abilities to replicate and establish persistence in immune cells. ADE can occur during the development of a primary or secondary viral infection, as well as after vaccination with a subsequent virus challenge. It has been observed mainly with positive-strand RNA viruses. Among them are Flaviviruses such as Dengue virus, Yellow fever virus, Zika virus, Coronaviruses, including alpha- and betacoronaviruses, Orthomyxoviruses such as influenza, Retroviruses such as HIV, and Orthopneumoviruses such as RSV.
A neutralizing antibody (NAb) is an antibody that defends a cell from a pathogen or infectious particle by neutralizing any effect it has biologically. Neutralisation renders the particle no longer infectious or pathogenic. Neutralizing antibodies are part of the humoral response of the adaptive immune system against viruses, intracellular bacteria and microbial toxin. By binding specifically to surface structures (antigen) on an infectious particle, neutralizing antibodies prevent the particle from interacting with its host cells it might infect and destroy. Immunity due to neutralizing antibodies is also known as sterilizing immunity, as the immune system eliminates the infectious particle before any infection takes place.
RV 144, or the Thai trial, is the name of an HIV vaccine clinical trial combining two vaccines that failed on their own, vaccinating in Thailand over the course of 24 weeks in October 2003 then testing for HIV until July 2006, publicly releasing efficacy findings in September 2009. The initial report shows that the rate of HIV infection among volunteers who received the experimental vaccine was 31% lower than the rate of HIV infection in volunteers who received the placebo. However, the reduction was not great enough for the Ministry of Public Health in Thailand to support approving the vaccine; it was reported in 2019 that it would have licensed it if the reduction had been 50% or more.
The STEP Study was a Phase IIb clinical trial intended to study the efficacy of an experimental HIV vaccine based on a human adenovirus 5 (HAdV-5) vector. The study was conducted in North and South America, the Caribbean, and Australia. A related study using the same experimental vaccine was conducted simultaneously in South Africa. These trials were co-sponsored by Merck, the HIV Vaccine Trials Network (HVTN), and the National Institute of Allergy and Infectious Diseases (NIAID), and had an Oversight Committee consisting of representatives from these three organizations. In South Africa the trial was overseen by the South African AIDS Vaccine Initiative.
HVTN 505 is a clinical trial testing an HIV vaccine regimen on research participants. The trial is conducted by the HIV Vaccine Trials Network and sponsored by the National Institute of Allergy and Infectious Diseases. Vaccinations were stopped in April 2013 due to initial results showing that the vaccine was ineffective in preventing HIV infections and lowering viral load among those participants who had become infected with HIV. All study participants will continue to be monitored for safety and any long-term effects.
MVA-B, or Modified Vaccinia Ankara B, is a particular HIV vaccine created to give immune resistance to infection by the human immunodeficiency virus. It was developed by a team of Spanish researchers at the Spanish National Research Council's Biotechnology National Centre headed by Dr. Mariano Esteban. The vaccine is based on the Modified vaccinia Ankara (MVA) virus used during the 1970s to help eradicate the smallpox virus. The B in the name "refers to HIV-B, the most common HIV subtype in Europe". It has been stated by Dr. Esteban that, in the future, the vaccine could potentially reduce the virulence of HIV to a "minor chronic infection akin to herpes".
SAV001-H is the first preventive HIV vaccine using a killed or "dead version" of HIV-1 virus.

HIV/AIDS research includes all medical research that attempts to prevent, treat, or cure HIV/AIDS, as well as fundamental research about the nature of HIV as an infectious agent and AIDS as the disease caused by HIV.
M. Juliana “Julie” McElrath is a senior vice president and director of the vaccine and infectious disease division at Fred Hutchinson Cancer Research Center and principal investigator of the HIV Vaccine Trials Network Laboratory Center in Seattle, Washington. She also is a professor at the University of Washington.
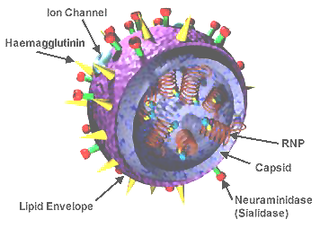
A universal flu vaccine is a flu vaccine that is effective against all influenza strains regardless of the virus sub type, antigenic drift or antigenic shift. Hence it should not require modification from year to year. As of 2019 there has been no approved universal flu vaccine for general use, but several have been in development.

Susan Zolla-Pazner is an American research scientist who is a Professor of Medicine in the Division of Infectious Diseases and the Department of Microbiology at Mount Sinai School of Medicine and a guest investigator in the Laboratory of Molecular Immunology at The Rockefeller University, both in New York City. Zolla-Pazner's work has focused on how the immune system responds to the human immunodeficiency virus (HIV) and, in particular, how antibodies against the viral envelope develop in the course of infection.
Intrastructural help (ISH) is where T and B cells cooperate to help or suppress an immune response gene. ISH has proven effective for the treatment of influenza, rabies related lyssavirus, hepatitis B, and the HIV virus. This process was used in 1979 to observe that T cells specific to the influenza virus could promote the stimulation of hemagglutinin specific B cells and elicit an effective humoral immune response. It was later applied to the lyssavirus and was shown to protect raccoons from lethal challenge. The ISH principle is especially beneficial because relatively invariable structural antigens can be used for the priming of T-cells to induce humoral immune response against variable surface antigens. Thus, the approach has also transferred well for the treatment of hepatitis B and HIV.
Hanneke Schuitemaker is a Dutch virologist, the Global Head of Viral Vaccine Discovery and Translational Medicine at Johnson & Johnson's Janssen Vaccines & Prevention, and a Professor of Virology at the Amsterdam University Medical Center of the University of Amsterdam. She has been involved in the development of Janssen's Ebola vaccine and is involved in the development of a universal flu vaccine, HIV vaccine, RSV vaccine and COVID-19 vaccine.









